Uncovering metabolic dysregulation in schizophrenia and cannabis use disorder through untargeted plasma lipidomics
December 28, 2024
Abstract
Cannabis use disorder affects up to 42% of individuals with schizophrenia, correlating with earlier onset, increased positive symptoms, and more frequent hospitalizations. This study employed an untargeted lipidomics approach to identify biomarkers in plasma samples from subjects with schizophrenia, cannabis use disorder, or both (dual diagnosis), aiming to elucidate the metabolic underpinnings of cannabis abuse and schizophrenia development. The use of liquid chromatography-high resolution mass spectrometry enabled the annotation of 119 metabolites, with the highest identification confidence level achieved for 16 compounds. Notably, a marked reduction in acylcarnitines, including octanoylcarnitine and decanoylcarnitine, was observed across all patient groups compared to controls. In cannabis use disorder patients, N-acyl amino acids (NAAAs), particularly N-palmitoyl threonine and N-palmitoyl serine, showed a strong downregulation, a pattern also seen in schizophrenia and dual diagnosis patients. Conversely, elevated levels of 7-dehydrodesmosterol were detected in schizophrenia and dual diagnosis patients relative to controls. These findings suggest a potential link between metabolic disruptions and the pathophysiology of both disorders. The untargeted lipidomics approach offers a powerful tool to identify novel biomarkers, enhancing our understanding of the biological relationship between cannabis abuse and schizophrenia, and paving the way for future therapeutic strategies targeting metabolic pathways in these conditions.
Introduction
Schizophrenia (SZ) is a chronic, disabling condition that typically manifests early in life, affecting up to 1% of the global population over a lifetime. The disease is associated with significant mortality and morbidity, leading to a reduced life expectancy of 10 to 15 years and no cure is available yet. Schizophrenia ranks as the seventh most costly illness worldwide, due to its early onset, frequent hospitalizations, the necessity for psychosocial support, and the associated loss of productivity1. While various hypotheses have been proposed regarding the aetiopathogenesis of SZ, none have been definitively validated. Although the symptomatic onset usually occurs in late adolescence or early adulthood, the disorder is rooted in genetic and/or environmental factors that are present long before symptoms emerge2.
Cannabis is among the most widely used substances globally, with an estimated 228Â million users aged 15 to 64. The risk of developing schizophrenia is significantly heightened with cannabis abuse, particularly when use begins at a younger age3. Additionally, approximately 10% of cannabis users are estimated to develop cannabis use disorder (CUD) over their lifetime. Interestingly, nearly one-third of individuals diagnosed with schizophrenia (SZ) have also been reported to meet the criteria for CUD4. However, the biological mechanisms that determine why some individuals develop schizophrenia while others experience only CUD, despite similar levels of cannabis exposure, remain unclear.
Regarding this, many metabolomic studies have tried to find specific biomarkers of schizophrenia for its early detection and disease progress monitoring. Different metabolic abnormalities have been found in patients with schizophrenia, but most studies highlight a different content of various amino acids and lipids compared to healthy subjects5,6,7,8,9. Similarly, cannabis users show distinctive metabolic profiles when compared to subjects that do not consume cannabis. In this sense, abnormal content of various lipids, amino acids, sugars, small proteins and other metabolites have been found in the plasma of cannabis users, suggesting that cannabis influences various systems and metabolic pathways besides the endocannabinoid system10,11. Within the afore-mentioned families of metabolites lipids can be highlighted due to their abundance in the brain and the key role of their metabolism for the functioning of central nervous system12,13,14.
The most widely used and best characterized biological matrices for clinical metabolomic studies are blood components, serum and plasma15. As blood is in contact with all organs and tissues, it allows monitoring of overall homeostatic changes in the body, and its collection is minimally invasive16. Nonetheless, plasma is usually preferred to serum in the search for potential biomarkers, as serum sampling presents a broader source of variability that can hinder comparisons between samples17.
In this context, schizophrenia and cannabis use may be reflected in the lipid composition through their association with neuroinflammation, oxidative stress and altered endocannabinoid signaling. Cannabis, in particular, disrupts endocannabinoid pathways18,19, which are lipid-derived signaling mechanisms that exacerbate or modify the symptoms of schizophrenia. By analyzing lipid profiles in the blood of patients with schizophrenia and cannabis use, we may gain insights into how cannabis interacts with pre-existing metabolic and neurochemical disturbances in schizophrenia.
Having such a background as a starting point, in this work, an untargeted lipidomic analysis of plasma samples from patients with schizophrenia, cannabis use disorder or comorbidity of both disorders was carried out.
Results
Annotation of untargeted non-polar metabolites
According to the workflow and data filtering described in the next section, a final list of 119 potential compounds in all the analyzed samples was obtained. Final candidates were annotated based on the endogenous metabolites mass lists (Human Metabolome Database and LipidMaps structure database) and MS2 mass spectra. The potential name of each candidate, grouped by compound classes, together with their predicted molecular formula, predicted monoisotopic mass and its error, the match level (with mzCloud spectra or with in silico fragmentation), retention time and the identification confidence level are shown in Supplementary Table S1. Lipids, predominantly fatty acids, glycerolipids, glycerolphospholipids and sphingolipids, represented the most detected class among all the potential candidates of the final list. Leaving aside 6 fatty acids that were annotated at level 2a due to high MS2 spectra matching and lack of ambiguity with other potential candidates, most of the identified lipids were annotated at level 3. The exogenous compounds (i.e. non-endogenous metabolites) are identified as “Others� in Table S2 under the “Categories/Subclass� column. They represent 19 of the 77 compounds included among the 2a/2b and 3 identification confidence levels. All the metabolites at level 3 (51% of candidates) showed acceptable spectral matches, but this is not enough to assure the discrimination between positional isomers or equivalent structures (e.g. the position of a double bond in unsaturated fatty acids), and therefore, only general structures and compound classes could be assured. Nevertheless, the putative name of the probable candidate (those with the higher number of citations) in metabolites annotated at level 3 was maintained. In contrast, candidates annotated at level 4 (35%), showed uninformative MS2 spectra and big ambiguity between other potential candidates, and thus, it was not even possible to determine the functional group or the class of these compounds. Consecutively, neither name nor family name was given to these compounds and only molecular formula was maintained.
The remaining candidates (13%) were annotated at level 2a or 2b due to unambiguous MS2 spectra matching with experimental MS2 available in mzCloud spectra or in silico fragmentation in Compound Discoverer, respectively. Within this group of compounds, three unsaturated fatty acids, two acyl carnitines, one N-acyl amine and one nicotinamide were identified. In addition to all the endogenous compounds mentioned above, some exogenous compounds and metabolites were identified at the same identification level, which deserve special attention. On the one hand, piperine (M003), tricyclazole (M012) and 2-hydroxybenzothiazol (M013) were identified, which have been detected in urine in other suspect analysis research works in the framework of human exposome assessment20. More importantly, active substances of drugs and pharmaceuticals and related metabolites have been found as well. In this sense, we can highlight, nicotine (M001) and cotinine (M002), as tobacco consumption biomarkers21; aripiprazole (M009) and dehydroaripiprazole (M010), as antipsychotic treatment biomarkers22, and 11-nor-9-carboxy-∆9-THC (M015), as cannabis consumption biomarker23.
Aripiprazole (Supplementary Fig. 1a) and dehydroaripiprazole (Supplementary Fig. 1b) were found in DUAL and SZ subjects but not in controls (for both metabolites and groups, log2(FC) = 5.5–8.8) or CUD subjects (for both metabolites and groups, log2(FC) = 2.9–3.1). Moreover, CUD and DUAL subjects had higher levels of nicotine and cotinine in plasma compared to controls (for both metabolites and groups, log2(FC) = 4.7–5.7) and to SZ patients (for both metabolites and groups log2(FC) = 1.2–2.1) (Supplementary Fig. 1). This finding suggests that there is a moderate tobacco consumption among CUD subjects (probably related to cannabis consumption as well) and DUAL subjects, and low or none tobacco consumption among SZ patients and controls. Higher 11-nor-9-carboxy-∆9-THC content was found in CUD subjects compared to the rest of groups (log2(FC) = 3.1 compared to DUAL, and, log2(FC) = 5.4–6 compared to SZ or controls) (Supplementary Fig. 1). These observations confirm that SZ and DUAL patients were under antipsychotic treatment, as well as cannabis consumption among CUD patients, and none consumption of any of these substances among controls. Although these expected findings were interesting to verify the consumption and treatment status of the subjects, these discriminative exogenous metabolites (together with the mentioned exogenous compounds belonging to exposome) directly related with the specific characteristic of the studied population were not taken into account for further analysis in order to go beyond the exposome analysis and study the alterations in lipidomic pathway.
Metabolic clustering of SZ, CUD and DUAL patients and their controls
Before analyzing the changes in the metabolic profiles of the three groups of patients, one of the first issues to be addressed was whether or not the recruited controls to each cohort (CUD, DUAL and SZ) were equivalent. To test this underlying hypothesis, control samples from different groups were compared by one way ANOVA using MetaboAnalyst (Supplementary Table S2). This analysis showed that 12 metabolites were significantly different in the three groups of control samples. Consequently, they were eliminated from all the data (together with the exogenous metabolites mentioned above) in order to avoid biased results.
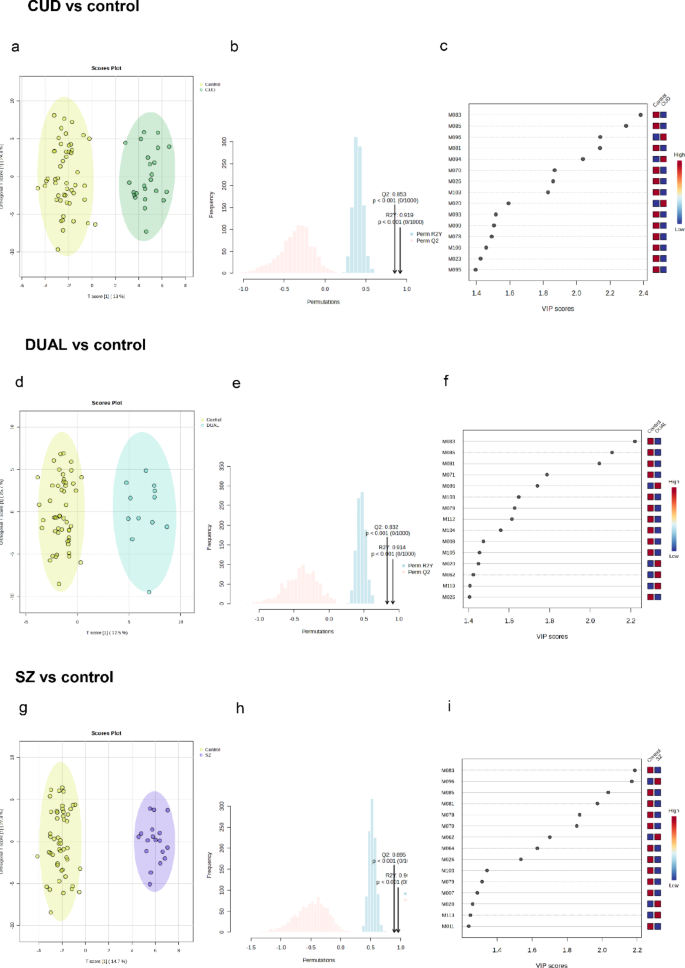
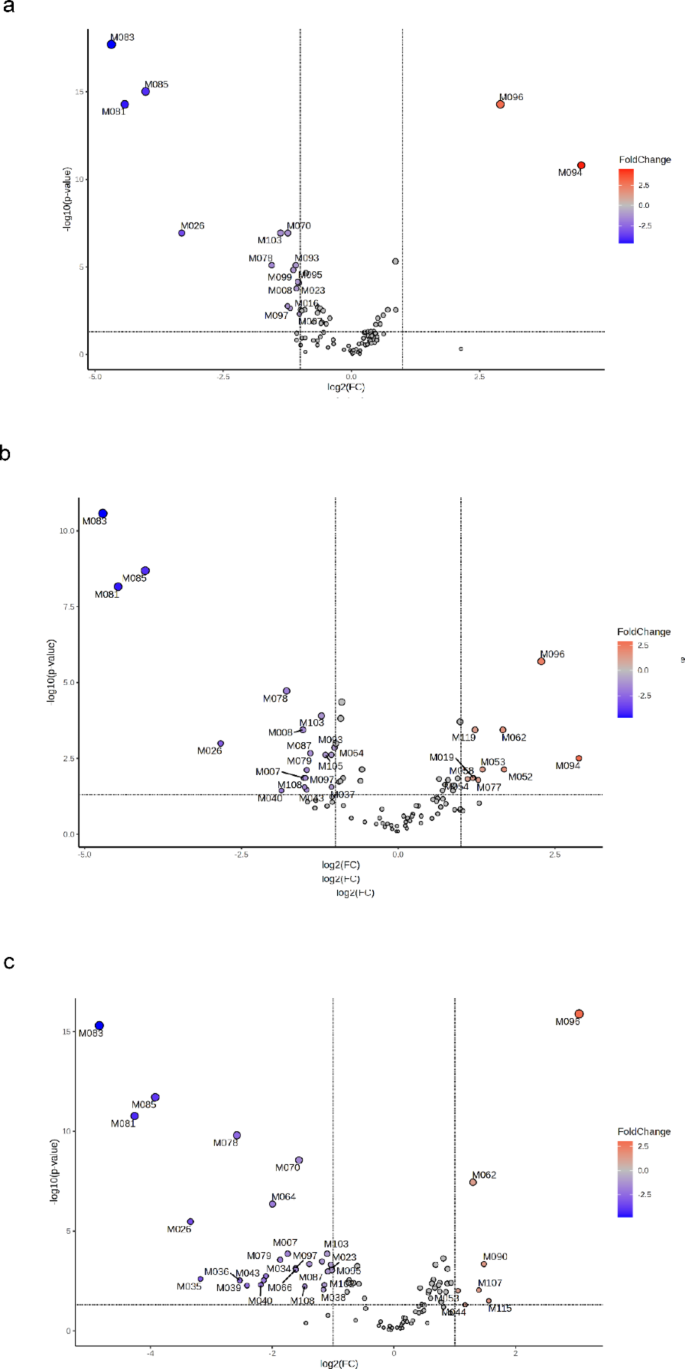
The metabolic profiles of each group of patients (CUD, DUAL and SZ) were then contrasted with those of the control group. Multivariate analysis using OPLS-DA was run along univariate analysis using FC and FDR t-tests. The score plots, permutation analysis results and the variable importance in projection (VIP) scores from the three OPLS-DA models are shown in Fig. 1. In addition, volcano plots obtained from univariate analyses are shown in Fig. 2.
OPLS-DA score plots showing metabolic clustering of (a) CUD (n = 24), (d) DUAL (n = 12) or (g) SZ (n = 18) patients compared to controls. Permutation analysis plotting RY2 and Q2 from 1000 permutation tests in the three OPLS-DA models (b) CUD (n = 24), (e) DUAL (n = 12) or (h) SZ (n = 18). Variable importance in projection (VIP) scores showing the first 15 metabolites that allowed discrimination of groups in the three OPLS-DA models (c) CUD (n = 24), (f) DUAL (n = 12) or (i) SZ (n = 18). Statistical significance was set at p < 0.05.
The score plots of the OPLS-DA models (Fig. 1a, d and g), show that the variability in the contents of the annotated metabolites allows metabolic clustering between control subjects and either CUD, DUAL or SZ patients. The cumulative R2Y and Q2 values of the OPLS-DA models were respectively, 0.92 and 0.85 for CUD vs. control; 0.91 and 0.87 for DUAL vs. control; and, 0.96 and 0.89 for SZ vs. control. Overall, around 40% of the variance was explained with the first two principal components. These values suggests that the OPLS-DA models fit the data correctly (R2Y near to 1) and that they have good predictive ability (Q2 > 0.5). Moreover, in the three OPLS-DA models, permuted RY2 values were around 0.6 or below, and most permuted Q2 values were below 0 (Fig. 1b, e and h). Also, permuted RY2 and Q2 values were in all cases below cumulative RY2 and Q2 values. All this suggests that the model fitting is adequate, and that was unlikely to be built by chance. After checking the suitability of the OPLS-DA models, metabolites that allowed discrimination between patient groups and controls were taken into account (Fig. 1c, f and i). To ensure a higher reliability of these results, among the potential biomarkers elucidated by the OPLS-DA analyses, only metabolites that showed significant changes according to volcano plots (Fig. 2) were further considered. Nonetheless, most of the metabolites that allowed discrimination of groups in the OPLS-DA models (those with the highest VIP) were significant according to volcano plots (FC > 2, p < 0.05), and vice versa.
Compared to controls, CUD subjects (Fig. 2a) presented a significant increase in the levels of two metabolites identified at level 4 (M094 and M096), and a significant decrease in the content of two acylcarnitines (M007 and M008), two N-acyl amino acids (M016 and M023), one primary amide (M026), one acetate ester (M070) and other nine metabolites (M078, M081, M083, M085, M093, M095, M097, M099 and M103).
DUAL (Fig. 2b) and SZ (Fig. 2c) patients, in contrast to CUD patients, presented a higher degree of metabolic impairments compared to controls. DUAL patients showed increased plasma levels of one oxo fatty acid (M019), four ceramides (M052, M053, M054 and M058), one sterol lipid (M062) and other four metabolites (M077, M094, M096 and M119); and decreased levels of two acylcarnitines (M007 and M008), one primary amide (M026), three glycerolphospholipids (M037, M040 and M043), one phenolic acid (M064) and other 11 metabolites (M078, M079, M081, M083, M085, M087, M093, M097, M103, M105 and M108). Similarly, SZ patients showed increased plasma levels of two ceramides (M044 and M053), one sterol lipid (M062) and other four metabolites (M090, M096, M107 and M115), along with decreased levels of two acylcarnitines (M007 and M008), one nicotinamide (M011), one N-acyl amino acid (M023), one primary amide (M026), seven glycerolphospholipids (M034, M035, M036, M038, M039, M040 and M043), one phenolic acid (M064), one dipeptide (M066), one acetate ester (M070) and other twelve metabolites (M078, M079, M081, M083, M085, M087, M095, M097, M099, M102, M103 and M108).
These analyses showed that either CUD, DUAL or SZ patients had distinctive metabolic profiles compared to healthy subjects, and pointed out a few potential biomarkers, some of them shared by two patient groups or more. It should be noted though, that some metabolites that underwent significant changes were among the metabolites identified at level 4. Indeed, a few of them (i.e., M081, M083, M085, M094 and M096) showed some of the highest scores (or highest FCs); being key compounds to provide metabolic clustering between patient groups and controls. Thus, although being potentially relevant metabolites, these metabolites identified at level 4 were not taken into account for further data interpretation owing to the lack of feasibility in their identification, which could lead to erroneous interpretations.
Plasma metabolic signatures in SZ, CUD and DUAL patients
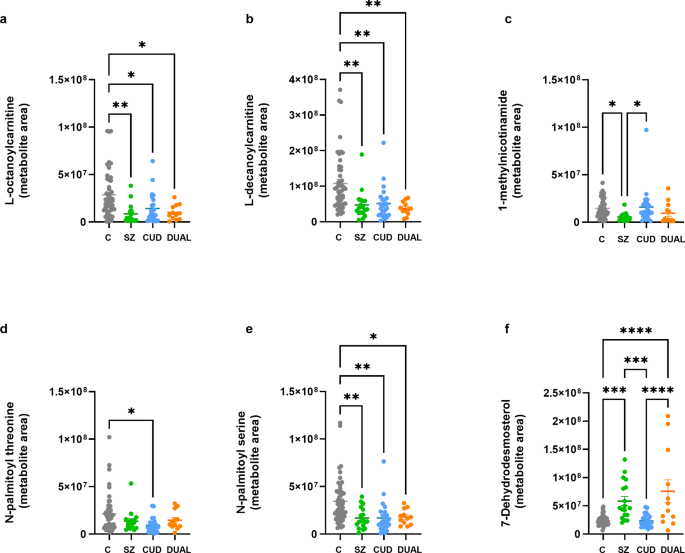
The metabolites differentially altered in the SZ, CUD and DUAL patients (Fig. 3, p values < 0.05, one-way ANOVA with Tukey’s post hoc test; see Supplementary Information for statistical data) were identified by inspection of the variable importance of projection (VIP) scores of the significant OPLS-DA models described above. One of the most relevant alterations is related with content of two acylcarnitines, L-octanoylcarnitine (Fig. 3a) and L-decanoylcarnitine (Fig. 3b), which are significantly decreased in the three groups of patients compared to control subjects. In addition, in SZ patients, the metabolite 1-methylnicotinamide (Fig. 3c) appears downregulated. The other metabolic impairments suffered by CUD patients, were the downregulations of the N-acyl amino acids (NAAAs) N-palmitoyl threonine (Fig. 3d) and N-palmitoyl serine (Fig. 3e), which was also downregulated in SZ and DUAL patients as well. In addition to the aforementioned changes, both SZ and DUAL patients showed a higher plasma content of a sterol lipid (7-dehydrodesmosterol) compared to control subjects (Fig. 3f).
Levels of discriminating metabolites selected by the OPLS-DA models in SZ (green, n = 18), CUD (blue, n = 24) and DUAL (orange, n = 12) groups versus controls (grey, n = 50). . Individual values and mean. One-way ANOVA with Tukey’s post-hoc corrections.*p < 0.05; **p < 0.005; ***p < 0.001; ****p < 0.0001.
Discussion
Many metabolomics studies have tried to find specific biomarkers of schizophrenia for its early detection and disease progress monitoring. Regarding this, mass spectrometry (MS) is the analytical technique of preference when it comes to clinical metabolomics of plasma and other biofluids15,24. Untargeted analysis allows to obtain a general metabolic profiling and eases the search of potential biomarkers, becoming the preferred approach for the preliminary steps in clinical metabolomic studies16,25. The results obtained from untargeted analyses have been further studied using both univariate and multivariate statistical analyses in order to seek for differences in the metabolic profiles of control subjects and patients from the different groups. In these sense, the relation between cannabis abuse and the development of schizophrenia from a metabolomic perspective is studied too, which could be useful for laying the groundwork for future metabolomic studies focused on the same concern.
Among the observed changes, the upregulation of ceramides and the downregulation of various glycerolphospholipids paired by the two groups individuals suffering from schizophrenia are worthy of particular attention.
Ceramides represent the central element in the metabolism of sphingolipids, ubiquitous constituents of eukaryotic cell membranes26. Besides their structural role, sphingolipids are involved in cellular signaling, cell differentiation and proliferation, apoptosis and inflammation, among other functions26,27. In the central nervous system, sphingolipids are located in neuronal cell membranes, and impaired sphingolipid metabolism has been linked with brain dysfunction and the development of different psychological diseases. Overproduction of ceramides results to be toxic and accelerates apoptosis of cells26. Additionally, increased content of different ceramides in brain tissues and blood has been related with neuroinflammation, altered synaptic transmission and accelerated apoptosis observed in various neurodegenerative and psychiatric disorders28,29. In this manner, compared to healthy subjects, increased levels of three ceramides and other three different ceramides have been quantified in postmortem white matter30 and plasma31 of patients with schizophrenia. These findings are in line with the observed upregulations of various ceramides in the plasma samples of patients of the SZ (M044, M053) and DUAL (M052-054, M058) groups in our study. In the case of schizophrenia, it has been proposed that the accumulation of ceramides may inhibit the action of excitatory amino acid transporter-2, leading to an accumulation of glutamate in the synaptic cleft, which results in excitotoxicity and hence, in neuronal damage32.
Glycerolphospholipids are the main constituents of neuronal and glial cell membranes, and are crucial to maintain the membrane’s structure and many membrane and cellular functions33. Phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs), comprise the most abundant classes of glycerolphospholipids in the brain. They are vulnerable to lipid peroxidation, and hence, the oxidative stress typically observed in schizophrenia has been related with an excessive breakdown of these membrane phospholipids, which leads to neuronal damage and brain dysfunction13,34,35. Besides, lysophosphatidylcholines (LPCs) and lysophosphatidylethanolamines (LPEs) are less abundant, but stand out due to their involvement in membrane function, apoptosis, and their protective role during oxidative stress and inflammation8. Therefore, a decreased content of these phospholipids may be also related with membrane dysfunction and a possible deficiency to face oxidative stress and neuroinflammation8,36. In line, with a suggested reduction of these key phospholipids in the brain, several studies have reported decreased content of various PCs, PEs, LPCs and LPEs in serum and plasma of patients with schizophrenia6,8,31,37,38 which is in accordance with our observations. Interestingly, these changes were mainly observed in SZ group subjects, where the content of three LPEs (M034, M035, M036), three PEs (M038, M038, M040) and one phosphatidylglycerol (PG, M043) were found to be significantly decreased compared to controls. Conversely, whereas subjects of the DUAL group only showed decreased levels of two PEs (M037, M040) and one PG (M043).
Related to the excitotoxicity, oxidative stress and neuroinflammation that patients with schizophrenia usually show39,40, it is worth mentioning the observed decrease in 1-methylnicotinamide (M060) levels in plasma samples of the SZ group. 1-methylnicotinamide is a neuroprotective endogenous metabolite that reduces glutamate excitotoxicity41, and can help to attenuate neuroinflammation, neuronal apoptosis42 and oxidative stress43. Therefore, a deficiency of 1-methylnicotinamide can ease the apparition of neuronal damage in case of glutamate accumulation, neuroinflammation and/or oxidative stress. In agreement with our results, Fan and colleagues reported a decrease of 1-methylnicotinamide in the plasma of patients with schizophrenia compared to healthy controls44.
One of the most relevant alterations was related with the content of two acylcarnitines, L-octanoylcarnitine and L-decanoylcarnitine, which were significantly decreased in the three groups of patients compared to healthy subjects. In the brain, acylcarnitines play a role in neuroprotection, lipid synthesis, membrane stability, gene and protein modulation, mitochondrial function, antioxidant activity, and cholinergic neurotransmission45. Altered acylcarnitine metabolism has been related with mitochondrial dysfunction, oxidative stress and inflammation46,47,48, processes that can lead to neuronal damage and that are usually found among schizophrenia patients49,50. In line with the observations found in this work, several studies have reported a decreased content of L-octanoylcarnitine and L-decanoylcarnitine in the plasma or serum of patients with schizophrenia, along with altered levels of other acylcarnitines17,51,52. Interestingly, Maayah and colleagues reported a lower content of L-octanoylcarnitine and medium- and short-chain acylcarnitines, and higher levels of some long-chain acylcarnitines in the serum of rats treated with THC-containing cannabis extracts, and proposed these as potential biomarkers of behavioral changes related to cannabis use53. Taking all this along with the findings of this work, we suggest that continued THC use could alter the regulation of acylcarnitine metabolism, exposing users to the psychological risks that these changes may entail.
Along with acylcarnitines, the other metabolic impairments suffered by CUD patients, were the downregulations of the N-acyl amino acids (NAAAs) N-palmitoyl threonine (M016) and N-palmitoyl serine (M023), which were also downregulated in SZ patients.
N-acyl amino acids (NAAAs) are an important family of endogenous signaling with relevant involvement in both physiological and/or pathological conditions54,55. These lipid mediators have been involved in energy homeostasis and neuroprotection56. Moreover, N-acyl amino acids can modulate glutamatergic and GABAergic neurotransmission57, key systems implicated in schizophrenia and addiction.
NAAAs share one inactivation enzyme (fatty-acid amide hydrolase, FAAH) and various molecular targets with endocannabinoids and, thus, they have been considered part of the “endocannabinoidome�58,59. The biosynthesis and physiological functions of some NAAAs, such as N-acylglycines, are well known; but, in general, NAAAs have only been identified and have not yet been studied in detail55,58,60. For instance, N-palmitoyl threonine (M048) was found to be upregulated in certain brain areas during induced acute inflammation in rats61, and the stearoyl NAAA with the same amino acid, N-stearoyl threonine, was found to have neuroprotective effects35. The neuroprotective action (through indirect action) of N-palmitoyl serine (Met_020) has been suggested62; and neuroprotective properties of other N-acyl serines have also been proposed35,63. Indeed, Wood et al., found elevated levels of some N-acylphosphatidylserines and N-acylserines (including N-palmitoyl serine) in postmortem frontal cortex of schizophrenia subjects16, proposing them as potential biomarkers of endogenous neuroprotective mechanisms.
Thus, the observed changes in plasma levels of the two NAAAs in subjects with CUD or SZ could imply a deficit in the neuroprotective mechanisms of these patients. However, due to the current lack of knowledge about the synthesis and biological implication of these NAAAs and the fact that fatty acids and amino acids are essential dietary elements55, more research is needed to properly interpret such metabolic changes.
The observed decrease in metabolite concentration in patients relative to controls may be indicative of underlying pathophysiological alterations associated with schizophrenia and cannabis use. Lipids and lipid-derived molecules play a pivotal role in neuroprotection, neurotransmitter modulation, and anti-inflammatory responses. A decrease of these metabolites may indicate an inability to maintain essential protective functions in the context of schizophrenia or cannabis use. Furthermore, diminished levels of key metabolites may also indicate mitochondrial dysfunction and energy deficiencies. Such changes are commonly observed in individuals with schizophrenia and are further exacerbated by the effects of cannabis on metabolic homeostasis64.
In addition to the aforementioned changes, both SZ and DUAL patients showed a higher plasma content of a sterol lipid (7-dehydrodesmosterol, M062) compared to healthy subjects. 7-dehydrodesmosterol is implied in the biosynthesis of cholesterol, neurosteroids and vitamin D3. A deficiency of either of these metabolites has been linked with an increased risk of psychosis65, hence it is difficult to correlate the up-regulation of 7-dehydrodesmosterol with the pathological state of patients with SZ or DUAL. However, it is worth mentioning that some antipsychotics66 as well as cannabis use66,67 may interact with lipid pathways. Aripiprazole (the antipsychotic mainly used by patients in the SZ and DUAL groups), inhibit the action of 7-dehydrocholesterol reductase, resulting in an increase of 7-dehydrocolesterol and 7-dehydrodesmosterol content65,68,69. Therefore, the observed increase in 7-dehydrodesmosterol levels in both SZ and DUAL patients may be a consequence of aripiprazole treatment, and thus, an indirect biomarker of its consumption.
The present work provides an additional evidence of the power of untargeted lipidomics for clinical studies. The LC-HMRS based analysis of plasma samples, followed by a proper treatment of chromatographic and mass spectrum data, allowed us to identify up to 119 unknown metabolites at different identification confidence level. Although many metabolites were identified merely at level 4 and obstructed the interpretation of some of the observed metabolic impairments, a considerable number of metabolites were identified between levels 2a and 3; which was enough to detect some key families of metabolites and other endogenous and exogenous compounds.
Multivariate and univariate analyses were key to find altered metabolic pathways among CUD, DUAL and SZ groups. The OPLS-DA models allowed clear discrimination between either of the groups of patients and healthy subjects; and pointed out some potential biomarkers. In addition, univariate analyses showed significant alterations of the same discriminant metabolites elucidated by OPLS-DA, which confers a higher level of confidence in the results and conclusions obtained. Some of the potential biomarkers elucidated from both analyses were identified at level 4, and thus, the metabolic pathways and biological implications related to these impaired metabolites could not be analyzed. Nonetheless, metabolic alterations of key metabolites’ families such as acylcarnitines, ceramides and glycerolphospholipids were found, which were in accordance to the literature and led to elucidate some preliminary hypotheses around the relationship between cannabis abuse and the risk of psychosis.
Overall, our findings highlight key metabolic disruptions, particularly the consistent reduction in acylcarnitines (L-octanoylcarnitine and L-decanoylcarnitine) across all patient groups, suggesting impaired fatty acid oxidation as a shared feature in both disorders. Additionally, the significant downregulation of N-acyl amino acids (N-palmitoyl threonine and N-palmitoyl serine) in CUD and schizophrenia suggests a disruption in lipid signaling pathways that may contribute to the pathophysiology of these conditions. The elevated levels of 7-dehydrodesmosterol in SZ and dual diagnosis patients point to altered cholesterol metabolism as a potential distinguishing metabolic signature.
These results provide insight into the biological mechanisms linking cannabis abuse and schizophrenia, offering novel biomarker candidates that could improve diagnosis and therapeutic strategies. While our findings underscore the utility of metabolomics in identifying metabolic pathways implicated in neuropsychiatric disorders, further research with larger cohorts is needed to validate these biomarkers and clarify their roles in disease progression. In conclusion, lipidomic profiling holds promise for advancing our understanding of schizophrenia and CUD, potentially informing targeted interventions that address metabolic dysfunction in these conditions.
Methods
Subjects
Subjects who met inclusion criteria for cannabis use disorder (CUD, n = 24), schizophrenia (SZ, n = 18), or dual diagnosis (DUAL, n = 12) (Table 1) according to DSM-IV/DSM-IV-TR70 were included in the study. The controls were voluntary donors who met the criteria for sex and age matching. The inclusion criteria for the controls were age 18–60, while the exclusion criteria included any use of cannabis or a diagnosis of a neuropsychiatric disease within the previous two years prior to blood extraction. All subjects were excluded if they met the criteria for a severe mental disorder other than schizophrenia or had a history of severe congenital, medical, or neurological illnesses. Moreover, a blood toxicological screening was performed in all subjects to determine the presence of antipsychotics as well as of THC67.
The demographic characteristics of the four subject groups used in the study as well as the antipsychotic treatment of all the cases are described in a previous work67 and in Supplementary Tables S3, S4 and S5. All the participants gave written, witnessed, informed consent for the participation in the study, that was approved by the corresponding Human Research Ethics Committee (University Cruces Hospital, code CEIC E14/43). All methods in the study were carried out in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice.
Plasma collection
Approximately 20 mL of blood was collected via venipuncture into ACD solution A Vacutainer® citrate blood collection tubes (Becton Dickinson & Company, Franklin Lakes, NJ, USA). The blood was drawn by nurses at the Drug Addiction Unit of the Uribe Mental Health Centre (Getxo, Spain), part of the Basque Health Service, and at the School of Medicine, University of the Basque Country (UPV/EHU) (Leioa, Spain).
For plasma preparation, blood cells were separated from plasma by centrifugation at 1,000–2,000 × g for 10 min in a refrigerated centrifuge set to 4 ºC. The plasma supernatant was then carefully transferred into a clean polypropylene tube using a Pasteur pipette. Samples were stored at -70 ºC until analysis, with the cold chain strictly maintained throughout collection and handling to preserve the integrity of chemical compounds.
Untargeted analysis of non-polar metabolites in plasma
Plasma samples were processed in order to extract mainly lipophilic metabolites and were subsequently analyzed with an untargeted approach by means of liquid-chromatography coupled to tandem high resolution mass spectrometry (LC-MS/HRMS). A detailed description of the employed materials and methods for the extraction of non-polar metabolites, LCMS/HRMS analysis, and data handling of the raw chromatograms has been compiled in Supplementary Methods File.
Briefly, a liquid-liquid extraction using methyl tert-butyl ether was carried out to extract non-polar metabolites from 0.5 mL aliquots of plasma samples. Organic phases were separated from the resulting biphasic mixtures, and were dried under nitrogen flow and reconstituted in xx mL of acetonitrile. Final extracts were analyzed along with instrumental blanks, procedural blanks and a pool of all studied samples as quality control (QC) sample in the LC-MS/HRMS system. Chromatographic separation of metabolites was carried out in reverse phase using a gradient chromatographic method, and targets were determined in both positive and negative ionization modes. The acquired raw chromatograms were filtered based on the adequacy of the chromatographic peaks, the mass accuracy and the quality of fragmentation spectra; and chromatograms of blanks and QC samples where used to correct potential analytical bias in sample processing and instrumental analysis. After data filtering, annotation of the final list of unknown metabolites was carried using freely available mass lists, and by contrasting the obtained fragmentation MS2 mass spectra with experimental fragmentation mass spectra libraries and in silico fragmentation. Identification confidence levels of the final candidates were done as proposed by Schymanski et al. (Schymanski et al., 2014).
Statistical analysis
To identify metabolites involved in altered metabolic pathways of the 104 individuals, the corrected areas determined in both sequences (i.e., positive and negative ionization modes) were merged in a spreadsheet treated in the same statistical analysis workflow using MetaboAnalyst 6.0 (MetaboAnalyst, n.d.). Data was logarithmically transformed and autoscaled and potential outliers were identified based on Principal Component Analysis (PCA). Equivalence of control groups was checked using one way ANOVA with Fisher’s post hoc test (p-value ≤ 0.05). Univariate and multivariate analyses were combined in order to find metabolic alterations and potential biomarkers in CUD, DUAL and SZ patients compared to healthy subjects. For univariate analysis, FC and t-tests (with p-value FDR correction) were used, applying a cutoff value of ≥ 2 for FC and cutoff value of ≤ 0.05 for p-values. For multivariate analysis, OPLS-DA was used and the quality of the models was evaluated based on R2Ycum (goodness of fit) and cumulated Q2cum values. Additionally, OPLS-DA models were validated using permutation tests (1000 permutations). For comparisons between the levels of discriminating metabolites selected by the OPLS-DA models in SZ, CUD and DUAL groups, ANOVA with Tukey’s post-hoc test was used.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
-
Ibrahim, H. M. & Tamminga, C. A. Schizophrenia: Treatment targets beyond monoamine systems. Annu. Rev. Pharmacol. Toxicol. 51, 189–209. https://doi.org/10.1146/annurev.pharmtox.010909.105851 (2011).
-
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97. https://doi.org/10.1016/S0140-6736(15)01121-6 (2016).
-
Kiburi, S. K., Molebatsi, K., Ntlantsana, V. & Lynskey, M. T. Cannabis use in adolescence and risk of psychosis: Are there factors that moderate this relationship? A systematic review and meta-analysis. Subst. Abus. 42, 527–542. https://doi.org/10.1080/08897077.2021.1876200 (2021).
-
Connor, J. P. et al. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 7, 16. https://doi.org/10.1038/s41572-021-00247-4 (2021).
-
da Silva, F., Zandonadi, E. A., dos Santos, F., Marques, M. S. & Sussulini, A. Metabolomics: A powerful tool to understand the schizophrenia biology. In Neuroproteomics as a Tool for Understanding Schizophrenia (ed. Martins-de-Souza, D.) 105–119 (Springer International Publishing, 2022). https://doi.org/10.1007/978-3-030-97182-3_8.
-
Davison, J., O’Gorman, A., Brennan, L. & Cotter, D. R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 195, 32–50. https://doi.org/10.1016/j.schres.2017.09.021 (2018).
-
Kopylov, A. T. et al. Consolidation of metabolomic, proteomic, and GWAS data in connective model of schizophrenia. Sci. Rep. 13, 2139. https://doi.org/10.1038/s41598-023-29117-7 (2023).
-
Liu, J., Xiu, M., Liu, H., Wang, J. & Li, X. Plasma lysophosphatidylcholine and lysophosphatidylethanolamine levels were associated with the therapeutic response to olanzapine in female antipsychotics-naive first-episode patients with schizophrenia. Front. Pharmacol. 12, 735196. https://doi.org/10.3389/fphar.2021.735196 (2021).
-
Song, M. et al. Potential plasma biomarker panels identification for the diagnosis of first-episode schizophrenia and monitoring antipsychotic monotherapy with the use of metabolomics analyses. Psychiatry Res. 321, 115070. https://doi.org/10.1016/j.psychres.2023.115070 (2023).
-
Alasmari, F. et al. Serum metabolomic analysis of male patients with cannabis or amphetamine use disorder. Metabolites 12, 179. https://doi.org/10.3390/metabo12020179 (2022).
-
Hinckley, J. D. et al. An approach to biomarker discovery of cannabis use utilizing proteomic, metabolomic, and lipidomic analyses. Cannabis Cannabinoid Res. 7, 65–77. https://doi.org/10.1089/can.2020.0002 (2022).
-
de San Roman, E. G. et al. CB(1) and LPA(1) receptors relationship in the mouse central nervous system. Front. Mol. Neurosci. 12, 223. https://doi.org/10.3389/fnmol.2019.00223 (2019).
-
Sethi, S. et al. Lipidomics, biomarkers, and schizophrenia: A current perspective. Adv. Exp. Med. Biol. 965, 265–290. https://doi.org/10.1007/978-3-319-47656-8_11 (2017).
-
Sethi, S., Hayashi, M. A., Sussulini, A., Tasic, L. & Brietzke, E. Analytical approaches for lipidomics and its potential applications in neuropsychiatric disorders. World J. Biol. Psychiatry 18, 506–520. https://doi.org/10.3109/15622975.2015.1117656 (2017).
-
Pedrini, M. et al. Advances and challenges in development of precision psychiatry through clinical metabolomics on mood and psychotic disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 93, 182–188. https://doi.org/10.1016/j.pnpbp.2019.03.010 (2019).
-
Wood, P. L. Mass spectrometry strategies for clinical metabolomics and lipidomics in psychiatry, neurology, and neuro-oncology. Neuropsychopharmacology 39, 24–33. https://doi.org/10.1038/npp.2013.167 (2014).
-
Liu, X. et al. Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal. Chim. Acta 1037, 293–300. https://doi.org/10.1016/j.aca.2018.03.009 (2018).
-
Cohen, K., Mama, Y., Rosca, P., Pinhasov, A. & Weinstein, A. Chronic use of synthetic cannabinoids is associated with impairment in working memory and mental flexibility. Front. Psychiatry 11, 602. https://doi.org/10.3389/fpsyt.2020.00602 (2020).
-
Little, R. & D’Mello, D. A cannabinoid hypothesis of schizophrenia: Pathways to psychosis. Innov. Clin. Neurosci. 19, 38–43 (2022).
-
Musatadi, M. et al. From target analysis to suspect and non-target screening of endocrine-disrupting compounds in human urine. Anal. Bioanal. Chem. 414, 6855–6869. https://doi.org/10.1007/s00216-022-04250-w (2022).
-
Benowitz, N. L., Hukkanen, J. & Jacob, P. Nicotine chemistry, metabolism, kinetics and biomarkers. In Nicotine Psychopharmacology (eds Henningfield, J. E. et al.) 29–60 (Springer Berlin Heidelberg, 2009). https://doi.org/10.1007/978-3-540-69248-5_2.
-
Kirschbaum, K. M. et al. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J. Biol. Psychiatry 9, 212–218. https://doi.org/10.1080/15622970701361255 (2008).
-
Huestis, M. A., Henningfield, J. E. & Cone, E. J. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH). J. Anal. Toxicol. 16, 283–290. https://doi.org/10.1093/jat/16.5.283 (1992).
-
Quintero, M. E., Pontes, J. G. M. & Tasic, L. Metabolomics in degenerative brain diseases. Brain Res. 1773, 147704. https://doi.org/10.1016/j.brainres.2021.147704 (2021).
-
Wishart, D. S. Metabolomics for investigating physiological and pathophysiological processes. Physiol. Rev. 99, 1819–1875. https://doi.org/10.1152/physrev.00035.2018 (2019).
-
Mencarelli, C. & Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. CMLS 70, 181–203. https://doi.org/10.1007/s00018-012-1038-x (2013).
-
Narayan, S. & Thomas, E. A. Sphingolipid abnormalities in psychiatric disorders: A missing link in pathology?. Front. Biosci. (Landmark. Ed.) 16, 1797–1810. https://doi.org/10.2741/3822 (2011).
-
Bernal-Vega, S., GarcÃa-Juárez, M. & Camacho-Morales, A. Contribution of ceramides metabolism in psychiatric disorders. J. Neurochem. 164, 708–724. https://doi.org/10.1111/jnc.15759 (2023).
-
Brodowicz, J., Przegaliński, E., Müller, C. P. & Filip, M. Ceramide and its related neurochemical networks as targets for some brain disorder therapies. Neurotox. Res. 33, 474–484. https://doi.org/10.1007/s12640-017-9798-6 (2018).
-
Schwarz, E. et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J. Proteome Res. 7, 4266–4277. https://doi.org/10.1021/pr800188y (2008).
-
Tkachev, A. et al. Lipid alteration signature in the blood plasma of individuals with schizophrenia, depression, and bipolar disorder. JAMA Psychiatry 80, 250–259. https://doi.org/10.1001/jamapsychiatry.2022.4350 (2023).
-
Ling, W. M., Chen, M. C., Zhang, Y. C., Ou, M. M. & Gu, L. Z. Study on ceramide modulates EAAT-2 participation in the immunoinflammatory response in schizophrenia. Eur. Rev. Med. Pharmacol. Sci. 23, 2263–2272. https://doi.org/10.26355/eurrev_201903_17275 (2019).
-
Fitzner, D. et al. Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. 32, 108132. https://doi.org/10.1016/j.celrep.2020.108132 (2020).
-
Yao, J. K., Leonard, S. & Reddy, R. D. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr. Res. 42, 7–17. https://doi.org/10.1016/s0920-9964(99)00095-x (2000).
-
Yao, L. Y. et al. Synthesis of Lipoamino acids and their activity against cerebral ischemic injury. Molecules 14, 4051–4064. https://doi.org/10.3390/molecules14104051 (2009).
-
Omori, W. et al. Reduced cerebrospinal fluid levels of lysophosphatidic acid docosahexaenoic acid in patients with major depressive disorder and schizophrenia. Int. J. Neuropsychopharmacol. 24, 948–955. https://doi.org/10.1093/ijnp/pyab044 (2021).
-
Costa, A. C. et al. Application of lipidomics in psychiatry: Plasma-based potential biomarkers in schizophrenia and bipolar disorder. Metabolites 13, 600. https://doi.org/10.3390/metabo13050600 (2023).
-
Li, M. et al. Impaired membrane lipid homeostasis in schizophrenia. Schizophr. Bull. 48, 1125–1135. https://doi.org/10.1093/schbul/sbac011 (2022).
-
Leza, J. C. et al. Inflammation in schizophrenia: A question of balance. Neurosci. Biobehav. Rev. 55, 612–626. https://doi.org/10.1016/j.neubiorev.2015.05.014 (2015).
-
Olloquequi, J. et al. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. (Oxford, England) 32, 265–275. https://doi.org/10.1177/0269881118754680 (2018).
-
Slomka, M., Zieminska, E., Salinska, E. & Lazarewicz, J. W. Neuroprotective effects of nicotinamide and 1-methylnicotinamide in acute excitotoxicity in vitro. Folia Neuropathol. 46, 69–80 (2008).
-
Mu, R. H. et al. 1-Methylnicotinamide attenuates lipopolysaccharide-induced cognitive deficits via targeting neuroinflammation and neuronal apoptosis. Int. Immunopharmacol. 77, 105918. https://doi.org/10.1016/j.intimp.2019.105918 (2019).
-
Tanaka, Y. et al. 1-Methylnicotinamide ameliorates lipotoxicity-induced oxidative stress and cell death in kidney proximal tubular cells. Free Radic. Biol. Med. 89, 831–841. https://doi.org/10.1016/j.freeradbiomed.2015.10.414 (2015).
-
Fan, Y. et al. Multi-omics analysis reveals aberrant gut-metabolome-immune network in schizophrenia. Front. Immunol. 13, 812293. https://doi.org/10.3389/fimmu.2022.812293 (2022).
-
Jones, L. L., McDonald, D. A. & Borum, P. R. Acylcarnitines: Role in brain. Progr. Lipid Res. 49, 61–75. https://doi.org/10.1016/j.plipres.2009.08.004 (2010).
-
Cuturic, M., Abramson, R. K., Breen, R. J., Edwards, A. C. & Levy, E. E. Comparison of serum carnitine levels and clinical correlates between outpatients and acutely hospitalised individuals with bipolar disorder and schizophrenia: A cross-sectional study. World J. Biol. Psychiatry 17, 475–479. https://doi.org/10.1080/15622975.2016.1178803 (2016).
-
Jamilian, H. et al. Oral carnitine supplementation influences mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Gynecol. Endocrinol. 33, 442–447. https://doi.org/10.1080/09513590.2017.1290071 (2017).
-
Kriisa, K. et al. Profiling of acylcarnitines in first episode psychosis before and after antipsychotic treatment. J. Proteome Res. 16, 3558–3566. https://doi.org/10.1021/acs.jproteome.7b00279 (2017).
-
Rajasekaran, A., Venkatasubramanian, G., Berk, M. & Debnath, M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 48, 10–21. https://doi.org/10.1016/j.neubiorev.2014.11.005 (2015).
-
Sertan Copoglu, U. et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 229, 200–205. https://doi.org/10.1016/j.psychres.2015.07.036 (2015).
-
Liu, Y. et al. Alteration of lipids and amino acids in plasma distinguish schizophrenia patients from controls: A targeted metabolomics study. Psychiatry Clin. Neurosci. 75, 138–144. https://doi.org/10.1111/pcn.13194 (2021).
-
Cao, B. et al. Serum metabolic profiling using small molecular water-soluble metabolites in individuals with schizophrenia: A longitudinal study using a pre-post-treatment design. Psychiatry Clin. Neurosci. 73, 100–108. https://doi.org/10.1111/pcn.12779 (2019).
-
Maayah, Z. H. et al. Metabolomic fingerprint of behavioral changes in response to full-spectrum cannabis extracts. Front. Pharmacol. 13, 831052. https://doi.org/10.3389/fphar.2022.831052 (2022).
-
Minarini, A. et al. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 13, 279–292. https://doi.org/10.1080/17425255.2017.1251580 (2017).
-
Battista, N., Bari, M. & Bisogno, T. N-acyl amino acids: Metabolism, molecular targets, and role in biological processes. Biomolecules 9, 822. https://doi.org/10.3390/biom9120822 (2019).
-
Long, J. Z. et al. The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166, 424–435. https://doi.org/10.1016/j.cell.2016.05.071 (2016).
-
Connor, M., Vaughan, C. W. & Vandenberg, R. J. N-acyl amino acids and N-acyl neurotransmitter conjugates: Neuromodulators and probes for new drug targets. Br. J. Pharmacol. 160, 1857–1871. https://doi.org/10.1111/j.1476-5381.2010.00862.x (2010).
-
Burstein, S. H. N-acyl amino acids (Elmiric Acids): Endogenous signaling molecules with therapeutic potential. Mol. Pharmacol. 93, 228–238. https://doi.org/10.1124/mol.117.110841 (2018).
-
Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 17, 623–639. https://doi.org/10.1038/nrd.2018.115 (2018).
-
Arul Prakash, S. & Kamlekar, R. K. Function and therapeutic potential of N-acyl amino acids. Chem. Phys. Lipids 239, 105114. https://doi.org/10.1016/j.chemphyslip.2021.105114 (2021).
-
Raboune, S. et al. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front. Cell. Neurosci. 8, 195. https://doi.org/10.3389/fncel.2014.00195 (2014).
-
Mann, A. et al. Palmitoyl serine: An endogenous neuroprotective endocannabinoid-like entity after traumatic brain injury. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 10, 356–363. https://doi.org/10.1007/s11481-015-9595-z (2015).
-
Cohen-Yeshurun, A. et al. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J. Cereb. Blood Flow Metabol. Off. J. Int. Soc. Cereb. Blood Flow Metabol. 31, 1768–1777. https://doi.org/10.1038/jcbfm.2011.53 (2011).
-
Roberts, R. C. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion 56, 91–101. https://doi.org/10.1016/j.mito.2020.11.009 (2021).
-
Korade, Ž et al. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 187, 74–81. https://doi.org/10.1016/j.schres.2017.02.001 (2017).
-
de Almeida, V. et al. Changes in the blood plasma lipidome associated with effective or poor response to atypical antipsychotic treatments in schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 101, 109945. https://doi.org/10.1016/j.pnpbp.2020.109945 (2020).
-
Ibarra-Lecue, I. et al. Cannabis use selectively modulates circulating biomarkers in the blood of schizophrenia patients. Addict. Biol. 27, e13233. https://doi.org/10.1111/adb.13233 (2022).
-
Balog, M., Anderson, A. C., Heffer, M., Korade, Z. & Mirnics, K. Effects of psychotropic medication on somatic sterol biosynthesis of adult mice. Biomolecules 12, 1535. https://doi.org/10.3390/biom12101535 (2022).
-
Genaro-Mattos, T. C. et al. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 24, 491–500. https://doi.org/10.1038/s41380-019-0368-6 (2019).
-
Gorgens, K. A. in Encyclopedia of Clinical Neuropsychology (ed J.S. Kreutzer, DeLuca, J., Caplan, B.) (Springer, 2011).
Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation (PID2019-106404RB-I00), Spanish Network for Stress Research RED2022-134191-T, Spanish Ministry of Health (PNSD 2019I021) and Basque Government (2019111082, ITIT1211-19, IT1512/22 and IT1446-22). Aitor Villate is also grateful to the UPV/EHU for the predoctoral fellowship. The authors thank the staff of the Uribe Mental Health Center (Osakidetza-Basque Health Service) for sample recruitment. The authors also thank technical and human support provided by Central Service of Analysis de Alava –SGIker� of UPV/EHU.
Author information
Authors and Affiliations
Contributions
L.U. and N.E. conceived and designed the study. L.F.C. collected the cohorts. P.U.L. and R.B.B. collected the samples. A.V. , M.O and A.U. collected the data and performed analyses. A.V and L.U drafted the manuscript. L.U, N.E and L.F.C. provided supervision and administrative support. All authors approved the final manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Villate, A., Olivares, M., Usobiaga, A. et al. Uncovering metabolic dysregulation in schizophrenia and cannabis use disorder through untargeted plasma lipidomics.
Sci Rep 14, 31492 (2024). https://doi.org/10.1038/s41598-024-83288-5
-
Received: 16 October 2024
-
Accepted: 13 December 2024
-
Published: 28 December 2024
-
DOI: https://doi.org/10.1038/s41598-024-83288-5
Keywords
Search
RECENT PRESS RELEASES
Related Post