Evaluating sodium-ion pouch cell battery for renewable energy storage under extreme condit
October 21, 2025
Abstract
A sodium-ion battery (SIB) is a sustainable energy storage technology based on abundantly available raw materials. It is a commercially viable option because of the processing similarity with Li-ion battery. Most of the energy storage studies focus on the near room temperature performance of different battery chemistries. Herein, we report the ultralow temperature performance of the SIB pouch cell. The cells fabricated using low-temperature compatible components showed significant specific energy values around 96, 74, and 46 Wh kg−1 at room temperature, −25 °C, and −50 °C, respectively. We demonstrated the battery performance under laboratory conditions as well as under actual windy and snowy environments. Such an exhibition highlights the use case of the SIB pouch cell as an emergency energy storage device in extreme weather conditions. Moreover, charging of SIB cell at −100 °C using polycrystalline Si solar cell is also reported, indicating the possibility of deployment for space expeditions.
Introduction
The increasing global awareness about climate change, critical resources depletion, and the need for sustainable energy solutions has imparted a shift towards renewable energy usage. Renewable energy sources, such as wind, solar, and hydropower, offer a clean and abundant alternative to fossil fuels, helping to reduce greenhouse gas emissions. However, the intermittent nature of these resources poses a challenge to maintain a reliable energy supply1. Therefore, energy storage technologies like batteries play a crucial role in the global energy transition. Batteries enable an efficient storage of the intermittent energy generated by renewable sources, thereby bridging the gap between energy generation and consumption. It is necessary to develop renewable energy systems that are resilient to extreme weather conditions. Widespread adoption of solar and wind energy is possible in the low-temperature regions. However, inefficiency of contemporary battery technologies at low temperatures may increase the cost of battery energy storage systems (BESS) substantially. Despite the higher costs, more than 100 Photovoltaic (PV)-BESS microgrid systems have been installed in Alaska, showcasing the significant potential in cold regions2. Similarly, over 900 gigawatts of wind power turbines are operational in Antarctica with sub-zero temperature conditions3. The effectiveness of these renewable energy sources also depends on the functional ability of the associated electrical infrastructure. Therefore, the development of high-performance, cost-effective, and durable battery systems suitable for all-weather conditions is necessary.
SIB is considered a more sustainable alternative to conventional Li-ion batteries (LIB) due to the high abundance and surface extraction of sodium compounds4. As emphasized by the International Energy Agency (IEA), critical minerals like copper, lithium, nickel, and cobalt are vital to the development of many rapidly advancing energy technologies, including wind turbines and solar cells5. The US Department of Energy (DoE) also recognized that, as the shift towards cleaner energy accelerates, the demand for these materials will increase rapidly6. Limited global reserves of critical minerals needed for LIBs and their energy-intensive extraction make sodium a promising option for sustainable energy storage7. SIBs have a minimum environmental impact and a short carbon payback period of 1.4 years as per the economic-ecological-efficiency analysis8. A lower production cost and fewer supply-chain constraints are important factors to meet the growing demand for sustainable energy storage9. A recent study from Stanford University, USA, suggests that SIBs can compete with low-cost lithium-ion variants by the 2030s10.
Recent advancements in cathodes and anodes have improved the efficiency and longevity of SIBs for energy storage11. Expanding these applications can enhance energy security for off-grid northern communities while lowering environmental impacts. In regions with cold climates, wind energy generation is abundant, but battery storage is not straightforward. Actual field demonstrations and performance data analysis from real-world systems are essential for validating the technologies and for enhancing the economic viability. There are several research articles on modifications in the cell chemistry to enable low-temperature operations of SIBs, mainly focusing on electrode/electrolyte engineering in coin cell format12,13,14,15,16,17,18,19,20,21. A recent study employing a THF-based weakly solvating co-solvent with hard carbon anodes investigated anion-rich solvation shells in half-cell configurations22. A practical prototype full-cell SIB has rarely been studied for very low temperature application23. A pouch cell assembly imitates the configuration of a commercial battery and the thermal insulation of the pouch cell casing can be added advantage for low-temperature energy storage.
Previously, we have reported low-temperature studies of different battery chemistries24,25,26,27. In addition to the anode/cathode materials, other battery components like electrolyte salt, solvent, separator, and the form factor are important for the low temperature measurements. Since sodium ion is an alternative to Li-ion chemistry and it is at an advanced stage of commercialization28, we intended to explore its performance at extremely low temperatures. We used a sodium-ion pouch cell that has potential for commercial up-scaling and deployment. The SIB pouch cell showed good performance for windmill energy storage from room temperature to –50 °C. In addition to laboratory conditions, we also demonstrated the in-field performance of the battery in a cold and windy climate. SIB can be a viable option for emergency power back-up during snow storms and extreme weather conditions. Moreover, we explored the possibility of SIB deployment for space expeditions or lunar missions where the temperature is lower and solar radiation is a readily available energy source. The storage of solar energy using SIB cell was studied at −100 °C. The SIB cell demonstrated efficient solar energy storage at such extremely low temperatures with specific energy ~70 Wh kg−1.
To our knowledge, this is the first practical evaluation of ultra-low temperature SIB pouch cells and their field demonstration for wind and solar energy storage, paving the way for building future-ready sustainable BESS for renewable energy.
Results & Discussion
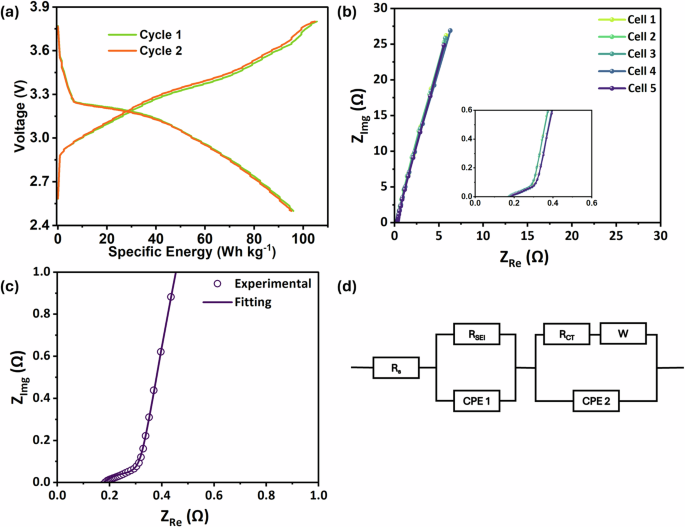
Various batches of SIB pouch cells were fabricated and tested for the present study. Figure 1a shows the galvanostatic charge discharge (GCD) curve of the SIB pouch cell tested at room temperature (~25 °C). The curve exhibits well-defined voltage plateaus during both charge and discharge processes, indicating stable electrochemical reactions. From this data, the cell achieves a specific energy of 96 Wh kg�¹, demonstrating its strong potential for practical energy storage applications. Electrochemical impedance spectroscopy (EIS) measurements were performed as shown in Fig. 1b, c to assess the electrochemical consistency and interfacial stability across multiple batches of SIB pouch cells. Figure 1b displays the Nyquist plots for these cells, revealing highly coinciding impedance spectra that are nearly identical across the samples. This overlap in the Nyquist plots indicates excellent reproducibility in cell fabrication and uniform electrochemical behavior. The close alignment of the plots suggests minimal variations in internal resistance and ion transport kinetics among the batches, underscoring the robustness of our fabrication process and the stability of the THF-based electrolyte system under room temperature conditions. Such consistency is crucial for practical applications, as it confirms reliable performance without significant batch-to-batch deviations, which could otherwise arise from inconsistencies in electrode coating, electrolyte filling, or assembly. Figure 1c illustrates the nonlinear curve fitting of the impedance data using EC-Lab’s Z-fit software, with the equivalent circuit model shown in Fig. 1d, to quantify the solid-electrolyte interphase resistance (RSEI) and charge transfer resistance (RCT) in the full cell configuration. Here, RS = 0.175 Ω is the total resistance of electrolyte, separator, and electrodes. The value of RSEI = 0.154 Ω is associated with the high-frequency semicircle in the impedance spectrum. The lower RSEI indicates the efficient formation and stability of the SEI layer on the hard carbon electrodes in the presence of THF-based electrolytes. RCT = 1.64 Ω along with the constant phase element (CPE 2) corresponds to the intermediate-frequency response of the cell, describing the distributed double-layer capacitance and resistance phenomena. Additionally, Warburg impedance (W) is indicative of the diffusion effect of Na+ ions at or on the porous electrode-electrolyte interfaces. The impedance data is consistent across multiple measurements, suggesting uniform SEI formation across all electrodes in these cells. This consistency indicates the reliability of the electrolyte and the reproducibility of electrode performance.
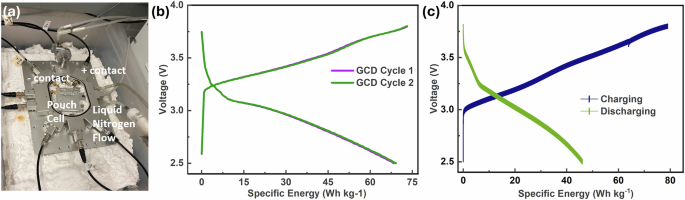
Considering the outstanding stability of the electrode and electrolyte used in the SIB pouch cells and the low freezing point of the solvents (THF, 2-MeTHF), we investigated the feasibility of these pouch cells for extremely cold environments on a customized system equipped with liquid nitrogen (LN2) cooling (ELTS). Figure 2a shows the SIB pouch cell connected between the customized cooling plates of ELTS. The temperature controller regulates the LN2 flow rate, thereby controlling the cooling plate temperature. Argon (Ar) gas is employed to purge the system of moisture, preventing frost buildup that could interfere with electrical measurements. The cooling plate probing station shows the placement of a pouch cell. The ELTS was wrapped and filled with Fiberfrax to further insulate the system and enhance LN2 efficiency. The SIB pouch cells were cycled at a voltage range of 2.5–3.8 V using an Arbin BT2000 cycler with Phoenix leads going into the customized cooling plate29,30.
Galvanostatic charge-discharge (GCD) studies were performed to evaluate the performance of SIB pouch cell at ultralow temperatures. The charging and discharging cycles of SIB pouch cell at −25 °C and −50 °C are shown in Fig. 2b and c, respectively. Discharge specific energy for the cell tested at −25 °C is around 74 Wh kg−1 at 1 C rate with a nominal voltage of 3.23 V. The discharge specific energy is around 46 Wh kg−1 at −50 °C, indicating excellent energy storage and delivery even at such low temperatures. The comparatively lower coulombic efficiency (CE) of SIB cells when tested at low temperature could be due to the kinetic limitations intensified by low temperatures. At lower temperatures, the viscosity of the electrolyte increases, slowing Na+ ion diffusion and de-solvation.
Rate capability assessments were conducted at 1 C and 2 C rates at −25 °C. The cells exhibited stable operation across this range with energy densities from 70 Wh kg−1 at 1 C and 30 Wh kg−1 at 2 C as shown in Supplementary Fig. S2. The battery’s durability and capacity retention over prolonged operation is evident from specific discharge energy of 28 Wh kg−1 after 100 cycles while maintaining ~88% retention compared to the 2nd discharge cycle at −25 °C (Supplementary Fig. S3).
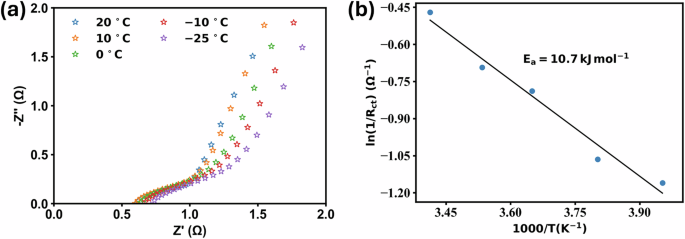
We have conducted a comprehensive analysis of sodium ion migration kinetics using temperature-dependent EIS data and Arrhenius modeling to quantify the impact of cold conditions on ion transport processes. The temperature-dependent Nyquist plots are shown in Fig. 3a. We used the Arrhenius equation to calculate the activation energy of ions migration-
Where, RCT is the charge transfer resistance, A is the pre-exponential factor, Ea is the activation energy for ion transport, R is the gas constant, and T is the absolute temperature. The calculated value of the activation energy from the Arrhenius plot shown in Fig. 3b is 10 kJ mol−1 for sodium ion transport in the THF-based electrolyte system. This low activation energy provides crucial insights into the low-temperature performance. The 10 kJ mol−1 activation energy is significantly lower than typical values reported for conventional carbonate-based electrolytes31, indicating more facile ion transport. The low Ea value explains why ionic conductivity remains considerable even at −100 °C.
However, the activation energy, while lower than carbonate systems, still results in a 7-fold increase from 1.6 to 12.18 Ω in RCT after low temperature testing as compared to RT. Consequently, polarization losses rise, leading to incomplete charging and reduced CE due to inefficient ion transport as observed in the low-temperature GCD from Fig. 2b, c. Nonetheless, the conductivity decrease with temperature follows a predictable Arrhenius relationship with minimal deviation. It indicates uniform functionality across the entire temperature range and stable electrode-electrolyte interface kinetics. The low activation energy directly correlates with sustained battery performance at low temperatures.
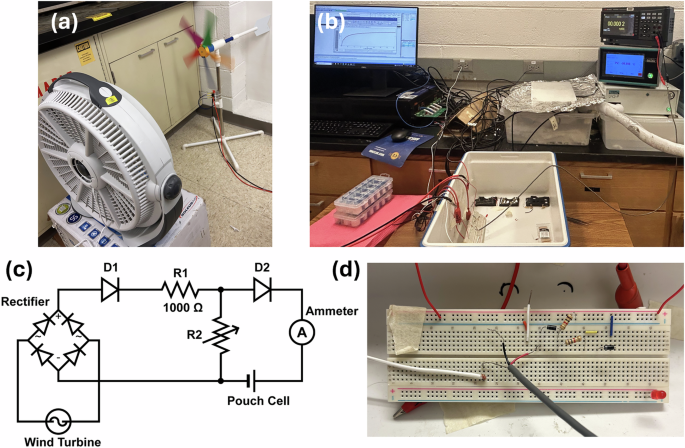
We demonstrated practical application of a low-temperature SIB pouch cell by charging it with a wind turbine at room temperature, −25 °C, and −50 °C. The real-world conditions for wind flow were mimicked in the laboratory by using, a table fan as a source of artificial wind generating device. As seen in Fig. 4a, wind blows when the fan is turned on, causing the turbine blades to rotate, making the rotor spin. This spinning motion is transferred to a generator, which converts the mechanical energy of the rotor into electrical energy via the principle of induction. This electrical energy is then used to charge the pouch cell. The rate at which the electrical energy is generated is given by the formula32
Where: P = Power output, watts; Cp = Power efficiency coefficient; � = Air density, kg m−3; A = Rotor swept area, m²; v = Wind speed, m s−1
The wind speed has a very high impact on the amount of power generated through the wind turbine. It may cause an issue, since too much current or voltage can damage the battery. A voltage divider circuit is designed to mitigate this issue by reducing the voltage provided to the battery. The integrated low temperature setup, schematic diagram of the voltage divider circuit, and the actual circuit on bread breadboard are shown in Fig. 4b, c and d, respectively. In the circuit, two resistors control the voltage and current being delivered to the battery. Adjusting the ratio between the two resistors allowed for control of the voltage, while adjusting the values of the resistors helped to control the current. Two Schottky diodes are also used in the circuit to prevent backflow of the current. The diode connected after the rectifier, D1, prevented the SIB pouch cell from discharging through the turbine, while the second one, D2, prevented the SIB from discharging through R2 once the battery is fully charged. A Schottky diode was specifically chosen over other diodes due to its lower forward voltage drop, which is kept at around 0.47 V for the entire range of charging currents used in this work. The equation given below is used to estimate the voltage being delivered to the battery. Small adjustments were required to account for factors such as internal resistances of the windmill and breadboard30.
Where- VB is Voltage given to the battery; Vw is voltage generated by the windmill; VD1 and VD2 are diode forward voltages and R1, R2 are registers.
The wind turbine generates AC electricity, which is converted into DC to charge the battery. This is achieved by connecting a simple rectifier to the wind turbine. A digital ammeter is connected between the diode D2 and the positive terminal of the SIB to measure the current. The SIB was placed in ELTS and was connected to 4 gold-plated leads. One pair was used to charge the pouch cell by the turbine, while the other was used to measure the voltage of the battery. The voltage measurement was done using an Arbin galvanostatic battery cycler.
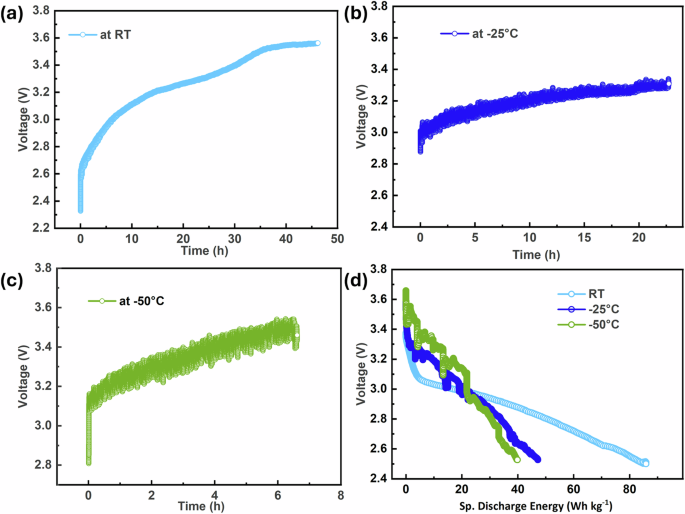
This experimental setup was used for the study of charging of SIB by windmill energy and discharging through the cycler at room temperature, −25 °C, and −50 °C. Figure 5a–c show the voltage vs time profile while charged with the wind turbine at room temperature, −25 °C, and −50 °C, respectively. Once the SIB is fully charged, it is manually disconnected from the charging circuit and is then discharged through the Arbin at a constant current. The discharge profiles of the cell at room temperature, −25 °C, and −50 °C are shown in Fig. 5d. The values of discharge specific energy are around 85, 47, and 39 Wh kg−1 for the room temperature, −25 °C, and −50 °C, respectively. Thus, it was demonstrated that the system worked for charging and discharging at low temperatures.
The cut-off voltages observed during different charging tests are not identical. This is a consequence of using passive charging setups (directly from windmill or photovoltaic cell sources) without active charge controllers, which reflects a minimalist renewable integration strategy.
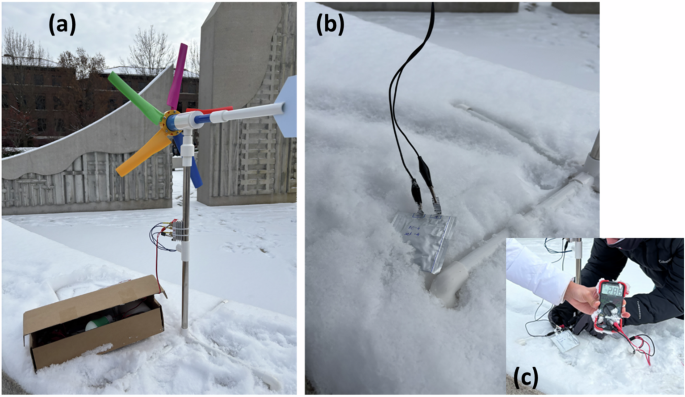
One of the objectives of the present work was to establish a use case of SIB for renewable energy storage in extreme temperature conditions. We demonstrated the wind energy storage in SIB pouch cells during winters at West Lafayette, Indiana, USA, when the temperature was around −10 °C, considering field operations. Figure 6a shows an LED light illuminated by wind energy. Figure 6b and c show an SIB connected with a wind turbine with a voltage of 2.86 V. It supports our laboratory measurements of the charging-discharging performance of SIB at very low temperature. In several regions on our planet, the temperature can be as low as –50 °C and the wind may be powerful enough to generate electricity. In extreme weather conditions during snowstorms and less sunshine, the combination of a windmill and SIB can be an alternative or emergency energy storage.
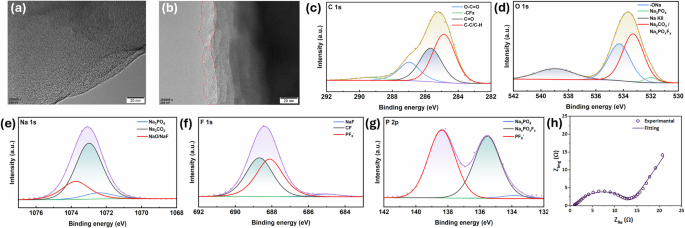
Post-mortem analysis was performed using Scanning Electron Microscopy (SEM), High Resolution Transmission Electron Microscopy (HRTEM), X-ray Photoelectron Spectroscopy (XPS), and EIS after the low temperature performance testing to investigate the anode’s interfacial stability and microstructural integrity. The SEM images revealed uniformity of the electrode surface in the ultra-low temperature (LT) tested samples compared to the pristine electrodes (Supplementary Fig. S4a–d). An excellent structural preservation with minimal degradation is evident from these images. Further insights into the robustness of the SEI formed on the anode surface is provided by HRTEM images. Figure 7a depicts the pristine HC electrode morphology with characteristic smooth edges typical of uncycled HC materials. Figure 7b shows the morphology of the post-testing sample, demonstrating the formation of a uniform and continuous SEI layer with a consistent thickness of 10–15 nm across the particle surfaces. This thin but uniform SEI layer under such extreme conditions reflects the stability of electrolyte decomposition products, preserves ionic conductivity pathways, and enables sustained electrochemical activity. There is a direct correlation with our findings of small increase in RSEI through impedance spectroscopy. The XPS survey scans (Supplementary Fig. S5) revealed that the pristine electrode has characteristic carbon and oxygen peaks with minimal surface contamination, whereas the LT tested electrode displays additional peaks corresponding to SEI constituents. The high-resolution spectra deconvolution for the LT tested electrode (Fig. 7c–g) reveals a multi-component interfacial layer comprising organic components derived from THF and 2-MeTHF electrolyte solvent decomposition (providing mechanical flexibility), inorganic content including NaF from NaPF6 salt decomposition (contributing to ionic conductivity pathways), and other mixed organic-inorganic phases.
After extreme low-temperature testing of the cell, EIS was performed to probe the changes in the system (Fig. 7h). The curve fitting of the obtained data showed an increase in overall impedance, with rises in RS, RSEI, and RCT (Supplementary Table S1), highlighting critical interfacial and kinetic degradation mechanisms. A significant rise in RCT likely originates from reduced charge transfer kinetics due to electrolyte viscosity increase and some salt precipitation at subzero temperatures. In addition, ultralow temperature operational conditions give rise to stress-induced microcracks and defects that may block ionic diffusion pathways. The altered low-frequency impedance response and a thickened SEI layer further amplifies interfacial resistance. However, it is important to note that the RSEI showed only a slight increase, which supports the effectiveness of the electrolyte system for ultralow temperature applications. An effective combination of suitable electrode materials with mix THF electrolyte can open a broad scope for SIBs in extreme environments.
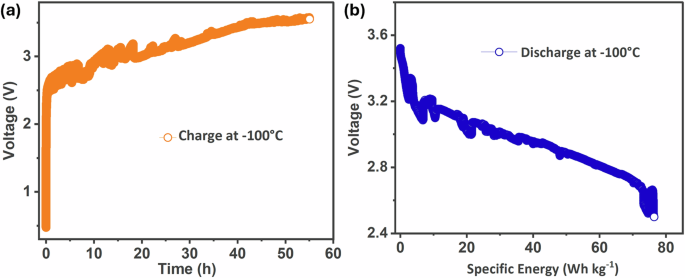
SIB’s utility can be extended further to space expeditions where the temperature is even lower at night and solar radiation is the prominent energy source during daytime. Typically, a lunar lander and rover can receive solar energy for 14 Earth days, but the next 14 Earth days are without sunshine and the temperature is around −100 °C. Under such conditions, an effectively performing battery pack is essential to power the rower and onboard electronic instruments. At least the battery can keep the machines in hibernation mode until the sunshine period begins again and the cycle continues. With such an application in sight, we investigated further the low-temperature performance of SIB in combination with a solar cell. The SIB pouch cell was maintained at -100 °C and was connected to a solar cell. The charging performance is shown in Fig. 8a. Even at such a low temperature, the SIB cell is charged optimally by the solar cell. Similarly, we also studied the discharge performance at the same temperature. It is evident from Fig. 8b that the SIB can dissipate up to 76 Wh kg−1 specific energy which is a significant value at extreme low temperatures.
The low temperature conditions affect the interface resistances as shown in Fig. 7h and Supplementary Table 1.
The observed fluctuations in the voltage curves during windmill and solar charging are due to the intermittent and variable nature of these power sources. These fluctuations reflect real-world renewable integration challenges. This study paves the way for future research in the area of ultra-low temperature energy storage devices.
In summary, SIBs represent a promising energy storage technology, yet their performance in extreme cold environments remains underexplored. We studied the performance of SIB pouch cell with ultralow temperature compatible components suitable for real time, field applications. The specific energy of the SIB cell was around 96 Wh kg−1 at room temperature. The ultra-low temperature measurements revealed specific energy values of 74 and 46 Wh kg−1 at −25 and −50 °C, respectively. The real-time deployment of the cells was showcased using charging of the cells by wind windmill at ultralow temperature using a specially designed setup, and in actual field conditions also. As an additional case study, the charging of SIB cells with solar cell at −100 °C was demonstrated, indicating SIB’s application portfolio even for space or lunar expeditions. These results highlighted the viability of SIBs for extreme environments while providing a framework for optimizing materials and architectures to mitigate low-temperature performance decay. While highlighting the robustness and practicality of the chemistry for off-grid renewable energy applications, this work lays important groundwork for future efforts involving scaled-up systems with commercial-grade inverters and power management solutions.
Methods
Low temperature SIB pouch cell fabrication
The anode was composed of hard carbon (HC) with 90 wt% active material, 5 wt% conductive additive, and 5 wt% binder. The cathode consisted of Sodium Vanadium Phosphate Na3V2(PO4)3 (NVP) with 85 wt % active material, 10 wt% conductive additive, and 5 wt% binder. Carbon coated aluminum foil was used as a current collector for the anode as well as the cathode. The mass loading was around 4 mg cm−2, and 10.5 mg cm−2 for the anode and the cathode, respectively. The coatings are dried at 80 °C in a vacuum oven overnight. The electrodes are cut to size (anode: 5.8 cm × 4.2 cm, cathode: 5.6 cm × 4 cm). Multiple layers of anode and cathode are stacked with a Polypropylene separator (Celgard 2500) and secured with adhesive tape. Aluminum tabs are welded to the electrodes, and the stack is enclosed in a polymer-coated aluminum foil pouch, sealed on three sides. The open side is filled with electrolyte in an Ar-filled glove box, followed by diffusion and Ar gas purging. Electrolyte is 1 M NaPF6 in Tetrahydrofuran (THF) and 2-methyl tetrahydrofuran (2-MeTHF) in a 1:1 ratio (v/v)
Finally, the pouch is sealed, completing the pouch cell fabrication as illustrated in the supplementary information Figure S1.
Electrochemical testing of pouch cells at low temperatures
Electrochemical impedance spectroscopy (EIS) was performed using an electrochemical workstation (Biologic VMP3). The software used for data acquisition and analysis was EC-Lab (version 11.33). EIS measurements were conducted at open circuit voltage (OCV) after a relaxation period of 24 hours. The frequency range was 500 kHz to 100 mHz, with a sinusoidal amplitude of 10 mV.
Galvanostatic charge/discharge (GCD) measurements were conducted on a battery testing system (Arbin LBT21084). The cells were cycled at a voltage range of 2.5 to 3.8 V at room temperature and low temperature of −25 °C, in a constant current mode for −50 °C. Below −25 °C the GCD of SIB pouch cell was conducted on a custom Extreme Low Temperature System (ELTS) equipped with liquid nitrogen cooling (INSTEC HCP402SG-PM+)29. Before low-temperature testing, the pouch cells were subjected to 2 cycles of charge and discharge at room temperature (25 °C).
Wind energy storage in SIBs at low temperatures
A small windmill generator (Nightbreeze) of 18 inches in diameter and equipped with a brushless motor and a rectifier circuit for AC-DC conversion is used as an energy source. The rated output voltage with load is 2.7-3.8 V. A consistent wind flow condition was mimicked in the laboratory by using a small table fan. Under experimental conditions, the output voltage without load is in the range of 10–12 V; therefore, a voltage divider circuit has been built to connect the SIB with windmill. Details of experimentation are further elaborated in the subsequent section.
Solar energy storage in SIB at very low temperature
The details of a specially designed set-up for ultra-low temperature performance testing of solar cells and battery cells have been reported previously30. A small photovoltaic cell (polycrystalline silicon, 5 V, 30 mA) and an SIB pouch cell were fixed in the cold chamber of the experimental setup. Both cells are at the same temperature with a continuous flow of liquid nitrogen (LN2) around the chamber. A top-side glass window allowed PV illumination with a light source to determine the open circuit voltage (VOC) and short-circuit current (ISC).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Jacobson, M. Z. On the correlation between building heat demand and wind energy supply and how it helps to avoid blackouts. Smart Energy 1, 100009 (2021).
-
Li, Q. et al. Enhancing battery energy storage systems for photovoltaic applications in extremely cold regions: A brief review. Energy Sustain. Dev. 81, 101517 (2024).
-
Global Wind Energy Council: GWEC’s Global Wind Report (2023).
-
Hwang, J.-Y., Myung, S.-T. & Sun, Y.-K. Sodium-ion batteries: present and future. Chem. Soc. Rev. 46, 3529–3614 (2017).
-
Global Critical Minerals Outlook 2024, International Energy Agency (IEA).
-
U.S. Department of Energy Critical Materials Assessment, (2023).
-
Singh, A. N. et al. Unleashing the potential of sodium-ion batteries: current state and future directions for sustainable energy storage. Adv. Funct. Mater. 33, 2304617 (2023).
-
Bai, H. & Song, Z. Lithium-ion battery, sodium-ion battery, or redox-flow battery: A comprehensive comparison in renewable energy systems. J. Power Sources 580, 233426 (2023).
-
Zhao, L. et al. Engineering of sodium-ion batteries: opportunities and challenges. Engineering 24, 172–183 (2023).
-
Yao, A., Benson, S. M. & Chueh, W. C. Critically assessing sodium-ion technology roadmaps and scenarios for techno-economic competitiveness against lithium-ion batteries. Nat. Energy 10, 404–416 (2025).
-
Wanison, R. et al. Engineering aspects of sodium-ion battery: An alternative energy device for Lithium-ion batteries, J. Energy Storage, 100, 113497 (2024).
-
Wang, C. et al. Extending the low-temperature operation of sodium metal batteries combining linear and cyclic ether-based electrolyte solutions. Nat. Commun. 13, 4934 (2022).
-
Shi, Q. et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries. Nat. Commun. 13, 3205 (2022).
-
Rui, X. et al. A low-temperature sodium-ion full battery: superb kinetics and cycling stability. Adv. Funct. Mater. 31, 2009458 (2021).
-
Li, Z. et al. Sodium-ion battery with a wide operation-temperature range from −70 to 100 °C. Angew. Chem. Int. Ed. 61, e202116930 (2022).
-
Wang, Q. et al. Fast-charge high-voltage layered cathodes for sodium-ion batteries. Nat. Sustain. 7, 338–347 (2024).
-
Zheng, X. et al. Knocking down the kinetic barriers towards fast-charging and low-temperature sodium metal batteries. Energy Environ. Sci. 14, 4936–4947 (2021).
-
Wang, X. et al. High-voltage aqueous planar symmetric sodium ion micro-batteries with superior performance at low-temperature of −40 °C. Nano Energy 82, 105688 (2021).
-
Wang, M. et al. Temperature-responsive solvation enabled by dipole-dipole interactions towards wide-temperature sodium-ion batteries. Nat. Commun. 15, 8866 (2024).
-
Zhu, Q. et al. A 110 Wh kg 1Ah-level anode-free sodium battery at −40 °C. Joule 8, 482–495 (2024).
-
Zhou, J. et al. Low-temperature and high-rate sodium metal batteries enabled by electrolyte chemistry. Energy Storage Mater. 50, 47–54 (2022).
-
Hengyi, F. et al. Regulating ion-dipole interactions in weakly solvating electrolyte towards ultra-low temperature sodium-ion batteries. Angew. Chem. 136, e202400539 (2024).
-
Bai, Z. et al. Low-temperature sodium-ion batteries: challenges and progress. Adv. Energy Mater. 14, 2303788 (2024).
-
Kim, S., Zhang, Y., Wang, H., Adams, T. E. & Pol, V. G. Enabling extreme low-temperature (≤ −100°C) battery cycling with niobium tungsten oxides electrode and tailored electrolytes. Small 20, 2306438 (2024).
-
Kim, S. & Pol, V. G. Tailored solvation and interface structures by tetrahydrofuran derived electrolyte facilitates ultra-low temperature lithium metal battery operations. ChemSusChem 16, e202202143 (2023).
-
Koppisetti, H. V. S. R. M. et al. Sustainable Enhanced Sodium-ion Storage at Subzero Temperature with LiF Integration. ACS Appl. Mater. Interfaces 15, 32291–32300 (2023).
-
Parekh, M. H. et al. Polysulfide shuttle mitigation through a tailored separator for critical temperature energy-dense lithium-sulfur batteries. Sustain. Energy Fuels 6, 5591–5599 (2022).
-
Wanison, R. et al. Engineering aspects of sodium-ion battery: An alternative energy device for Lithium-ion batteries. J. Energy Storage 100, 113497 (2024).
-
Jamison, C. M., Kim, S., Ramasamy, H. V., Adams, T. E. & Pol, V. G. Lithium-Ion battery testing capable of simulating ultralow lunar temperatures. Energy Technol. 10, 2200799 (2022).
-
Adams, E. et al. Efficient photovoltaics integrated with innovative li-ion batteries for extreme (+80°C to −105°C) temperature operations. Sci. Rep. 15, 10190 (2025).
-
Eshetu, G. G. et al. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 10, 2000093 (2020).
-
Anaya-Lara, O., Jenkins, N., Ekanayake, J., Cartwright, P. & Hughes, M., Wind energy generation: modelling and control, (Wiley, 2009).
Acknowledgements
VGP sincerely thanks Program Manager Dr. Corey Love for his support through the Naval Enterprise Partnership Teaming with Universities for National Excellence (NEPTUNE) program, Office of Naval Research (Grant #N000142412510), for the project ‘Understanding the Role of Electrode-Electrolyte Interphases at Ultra-low Temperature Li-ion Batteries for Prototype Development’.
Author information
Authors and Affiliations
Contributions
M.S.- Conceptualization, Strategy, Investigation, Performing experiments, Data analysis, Writing−original draft; J.B.- Assistance for low-temperature measurements; V.M.- Pouch cell fabrication, Data Analysis, assistance for manuscript writing; V.P.- Conceptualization, Strategy, Supervision, Manuscript editing, Funding acquisition, and Communication.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests
Peer review
Peer review information
Communications Chemistry thanks Zhicheng Ju, Zachary Gossage, and the other anonymous reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shelke, M., Boyer, J.M., Mirzapure, V. et al. Evaluating sodium-ion pouch cell battery for renewable energy storage under extreme conditions.
Commun Chem 8, 315 (2025). https://doi.org/10.1038/s42004-025-01709-6
-
Received: 10 June 2025
-
Accepted: 12 September 2025
-
Published: 22 October 2025
-
DOI: https://doi.org/10.1038/s42004-025-01709-6
Search
RECENT PRESS RELEASES
Related Post