Building a clean-energy future, brick by brick
November 13, 2025
A house constructed from red “smart bricks” charged to provide electricity. A hospital operating room built with self-cleaning tiles that kill superbugs. Semiconductor nanoparticles to improve MRIs or generate clean water. Micro-batteries embedded in clothing to monitor your health.
These are a few of the applications that could emerge from the foundational research occurring in the lab of Professor Julio D’Arcy, the Carl J. and Anna Carlson Endowed Chair of Chemistry and Biochemistry.
“We’re really a material science, engineering, and chemistry lab that works at the intersection of renewable energy, climate change, and health,” D’Arcy says. “We live in that space.”
A $500,000, five-year National Science Foundation CAREER Award is funding D’Arcy’s study of the interplay of aerosol formation and vapor phase chemistry in forming organic semiconductor nanoparticles.
His research could be used to lower costs for energy storage in devices or enable development of lightweight batteries for transportation applications.
“Everyone’s probably heard stories about how everything ‘nano’ is better,” he says. “Physics change at that smaller scale, and things behave better. Ion transport is facilitated at this scale, enabling rapid charging.”

Many days, D’Arcy’s lab is abuzz with the activities of 17 undergraduate and graduate students who work with iron oxide, or rust, which makes bricks red. They develop and study synthetic polymers — complex, chemically bonded chains of large molecules — used to produce materials for energy storage devices, atmospheric water harvesting, pH and temperature sensors, and other applications, including those in medicine.
“We’re always after the same goal,” D’Arcy says. “We want to make a polymer that conducts electricity. The more it conducts, the better.”
After his brick research was published by Nature Communications in 2020, D’Arcy heard from hundreds of private companies, global investors, and journalists interested in his work. As he told Scientific American, “We’re trying to make specialized plastics that are only used on the nanoscale — where we use very little of the plastic, and we can actually embed that plastic inside construction materials.”
His research team didn’t expect such worldwide attention. “We got a little taste of what can happen if you publish in a field where people care about the intersection of this research,” recalls D’Arcy, who joined Clark’s Gustav H. Carlson School of Chemistry and Biochemistry in 2022. “They’re all after the same idea: adding sustainability into construction materials. It’s not just the architecture they’re interested in. It’s the idea that construction materials are going to make you live longer, or the idea that you’re going to be able to store energy in bricks, steel, or concrete.”

D’Arcy’s synthesis of organic semiconductor nanoparticles would fill a commercial and societal need. “It’s something that manufacturers want,” he says. “We propose a method that produces nanoparticles very fast, using aerosols. It’s hard to do, but it’s doable.”
Meanwhile, his research on developing smooth polymers to create antibacterial coatings also holds much promise.
“You could use this masonry material in operating rooms or kitchens,” D’Arcy says, “anyplace where you have people with compromised immune systems or where you prepare food.”
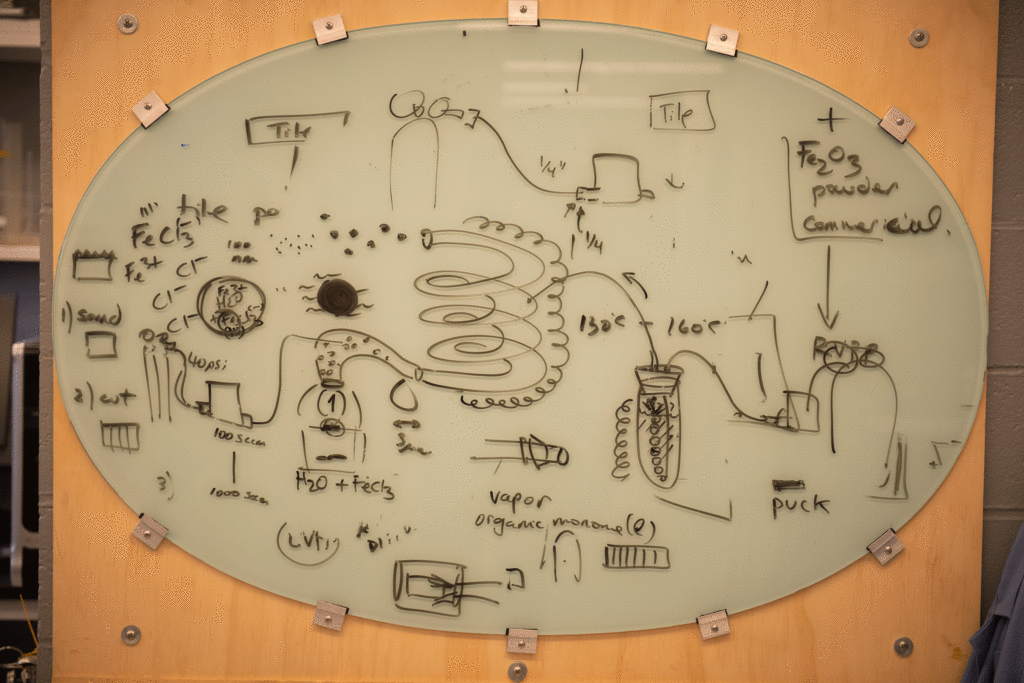
To form nanoparticles of a polymer called PEDOT — short for Poly(3,4-Ethylenedioxythiophene) — D’Arcy’s students design and build glass-and-metal, box-shaped reactors in which they place 1-centimeter-square brick samples. They fill the reactors with ferric chloride, an acid in water.
“We’re heating the sample and exposing it to vapors or reactants,” D’Arcy explains. “The monomer, or simple molecule, gets polymerized on the surface of the brick once it’s been activated by the acid.”
The shape of the polymer determines its application. “We make nanotubes, nanofibers, and smooth films,” he says.
Besides contributing to foundational research that could result in wide-ranging applications, students in D’Arcy’s lab are gaining hands-on, career-ready experience and preparing to publish journal articles.
They include two undergraduate students, Avery Schwartz ’26 and Ella Traynor ’26, who have studied chemical reactions that produce polymers and their semiconductor nanoparticles.
A double major in chemistry and art history, Schwartz has worked in the lab for more than a year, funded last summer by a Weiller Research Fellowship. She has presented twice at ClarkFEST, the biannual undergraduate research and scholarship event.

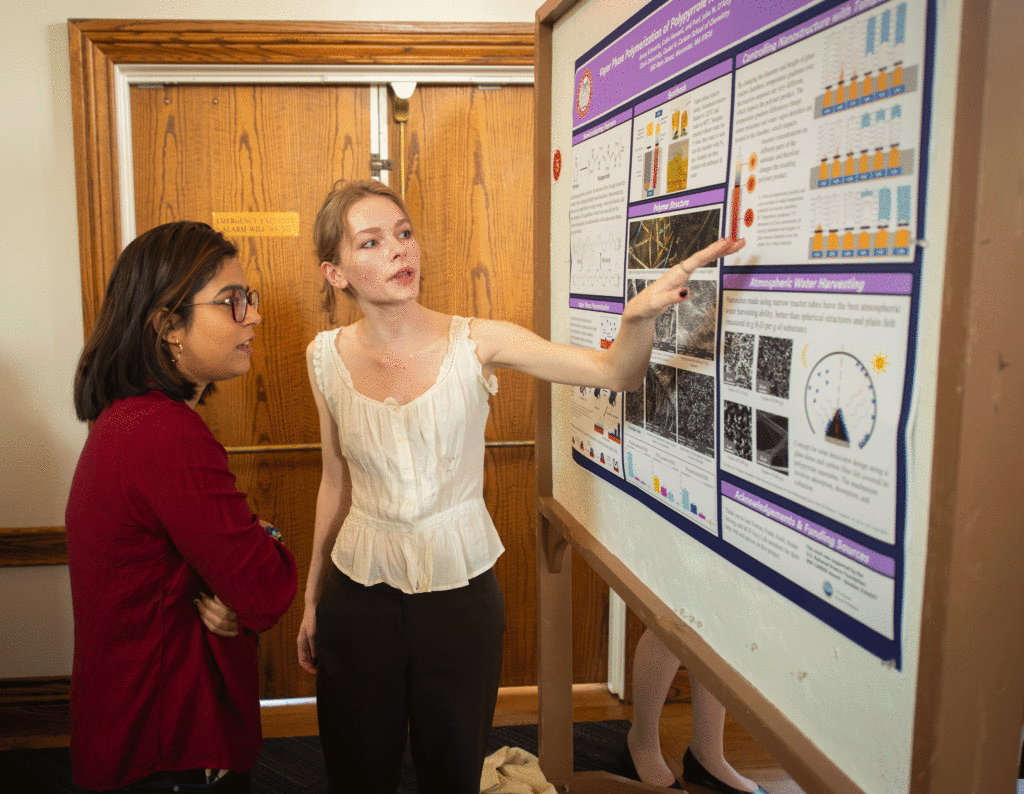
Schwartz’s research examines the chemical reaction that occurs when nanotube structures — which can attract, absorb, and hold atmospheric water — are formed during the “vapor phase” of polymerization. She studies nanotube “films” of polypyrrole, a type of polymer that has applications for energy storage, water purification, and water storage.
Although Schwartz investigated the formation of polymer films on glass, “you can apply the polymer film to any substrate, like a brick, and collect even more water,” she says, benefiting areas of the world that need clean drinking water.
In the lab, Schwartz’s two academic majors “play off of each other in an interesting way,” she says. “Art history is a lot about noticing things and taking the time to craft and engineer an argument about whatever work you’re talking about or evidence you’re seeing in the history. And I think that’s what makes for a successful chemist as well — deeply analyzing a lot of the work that you’re doing and the observations you’re making.”


Funded over two summers — a Murdock Research Fellowship in 2025 and a Penn Family Research Fellowship in 2024 — Traynor has worked in D’Arcy’s lab for nearly three years, “doing a little bit of everything.” Traynor’s main focus, however, is studying how and why the introduction of a power source during the vapor phase synthesis of PEDOT results in a chemical reaction that forms a germ-killing peroxide.
The research could be applied to developing self-sterilizing brick tiles for hospital operating rooms.
“It’s like my research has come full circle.”
— ella traynor ’26
“There’s the water-splitting reaction caused by the battery and our polymer, and then there’s the iron oxide in the bricks, which results in a chemical, or Fenton, reaction,” they explain, “and that’s what results in those peroxide radicals that are so good at killing organisms like bacteria.”
A chemistry major who also enjoys and hopes to minor in physics, Traynor now is mentoring another undergraduate student, Beckham Ward ’28, who just joined D’Arcy’s lab. “I was showing him how to do stuff with my project. That was cool — it’s like my research has come full circle.”
A catcher and infielder for women’s softball, Traynor made the 2025 Softball Academic All-Conference Team last spring. They plan to continue their research next year as part of Clark’s 4+1 Accelerated Master’s Degree in chemistry.
Juggling academics, softball practices, and games as a student-athlete has forced them to “stay organized. It’s given me a lot of time-management skills,” says Traynor, who also applies their coach’s advice to apply a “growth mindset” to build her softball skills. “It makes me a little more focused on moving forward or what I need to do next.”
Playing catcher behind home plate also has provided Traynor with additional skills they can apply to chemistry research.
“You’re thinking through plays. You’re the only one who sees the whole field,” Traynor says. “People are listening to you because you’re the only one who has eyes on all the runners and all the fielders. I think that helps you be a little more assertive. You must be a quick thinker, you must be decisive, and you must have a plan. And I think that applies to real life and the lab as well.”

Both Schwartz and Traynor have benefited from mentoring by graduate students in the lab, including current master’s student Yiming Qin and Ph.D. students Umama Akther and Ye Chen.
Qin’s research and master’s thesis focus on micro-supercapacitors, the conducting polymers that can serve as tiny batteries to store energy.
“I’m working with carbon fiber paper,” he says. “Carbon fiber paper is a substrate, and we grow our conducting polymer PEDOT nanofibers on it.”
Although carbon fiber is fragile, it can become stronger when woven together to cover a larger area, according to Qin.
“It could be used like a wearable, like energy storage devices in smart clothes, smart shoes, or smart watches,” he says.

Qin is working with D’Arcy to publish his research, then hopes to return to China to work in industry and care for his parents.
When Akther was applying to chemistry Ph.D. programs in the United States, her mentors and peers from Bangladesh urged her to apply to Clark, she says.
As an undergraduate student in chemistry at Jashore University of Science and Technology, Akther had had worked in a research lab on a project to improve food safety. She studied the computational chemistry behind using simple salt or vinegar solutions to remove heavy metals from vegetables grown in Jashore for sales and export. The research resulted in a recent article published in the Journal of Food Composition and Analysis.
Akther wanted a similarly robust research experience in a Ph.D. program. When D’Arcy offered her a position in his lab, her supervisor in Bangladesh suggested she take it.
“She said, ‘This project is really, really good. This is a promising field for industry and jobs, and you can work in the field — polymer chemistry, nano chemistry, and also engineering,’ ” Akther recalls.



Now Akther is in her second year at Clark, leading her own research project and mentoring undergraduate students in D’Arcy’s lab. With assistance from undergraduate students Katelynn Humphrey ’25 last year and Emme Salisbury ’28 and Emma St. Louis ’28 this year, Akther has sought to develop and build a reactor that uses aerosol vapor polymerization. The goal, she says, is to more rapidly — and easily — produce smaller organic semiconductor nanoparticles of PEDOT, shaped like spheres for easier delivery.
Her research has potential biomedical applications. “If we make this particle at the nanometer-size scale,” she says, “then it can be inserted into our bodies because it’s safe — it has no side effects. It can be used in MRI imaging.”
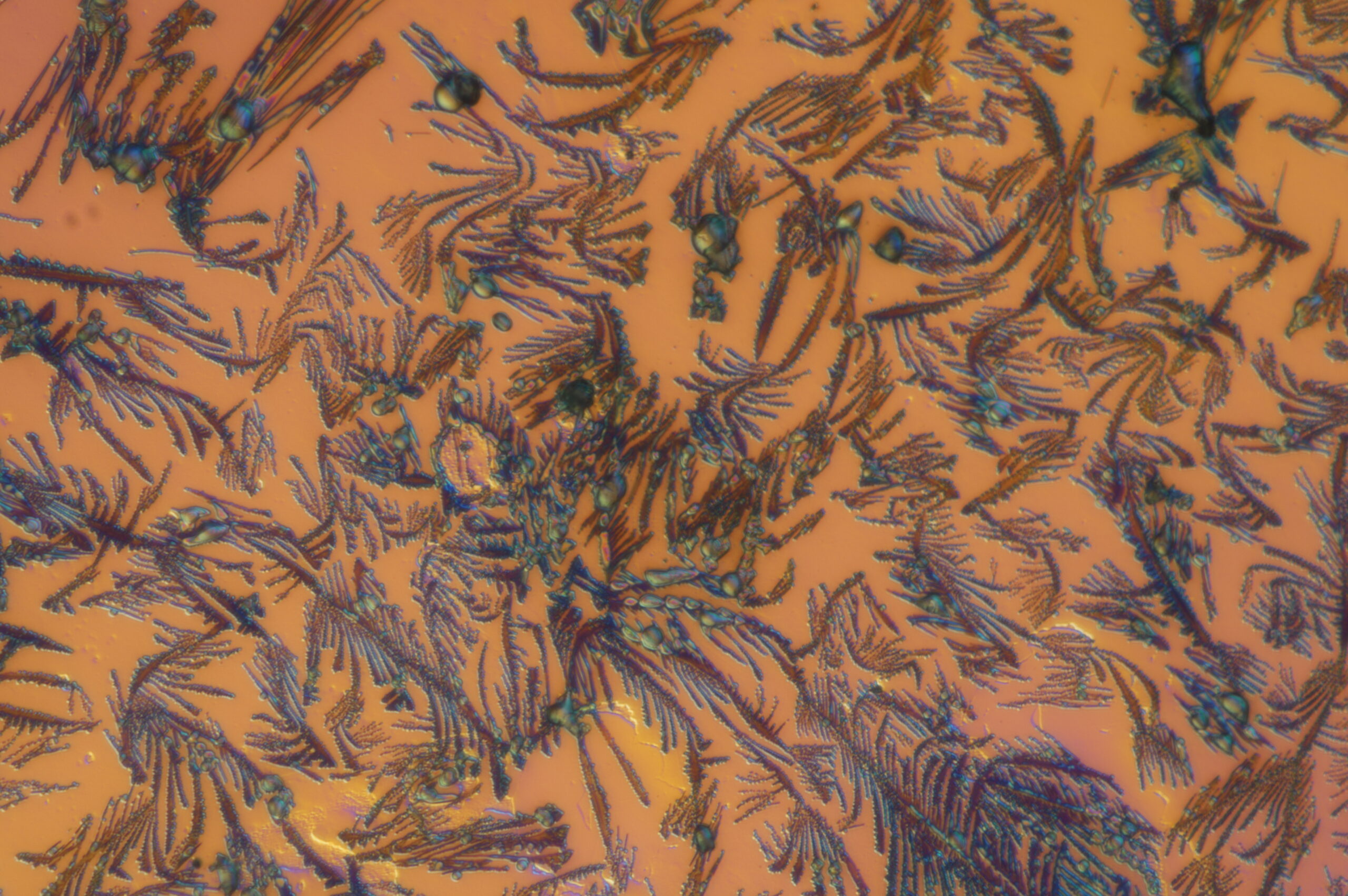
Yet, the work can be slow and tedious at times. “Over a year, I have tried eight to nine times to change my reactor. I need a more advanced reactor so it can handle more nanoparticles,” she says.
Akther uses imaging software to examine the microscopic nanoparticles. “If I’m getting 9-micrometer particles, but my goal is 5 micrometers,” she explains, “then I think, ‘OK, what can I change to make my particle smaller? Also, how many am I making? Are the particles attached to each other? What is the problem within my reactor?’ ”
She is overseeing another undergraduate student, Tatsuya Peralta ’27, a chemistry major who is helping her build more powerful nebulizers to produce aerosolized droplets of ferric chloride, which are carried into her reactors. They will replace the store-bought household humidifier she has been using.
“I can produce a hundred particles in one run now,” she explains. “But if my humidifier is more powerful, then I can make 200 or 300.”
In the field, research exploring aerosol polymerization has not been widely explored, Akther says, “so we would like to introduce this process to others.”
Even more, D’Arcy says, if he and his research team make inroads in their polymer studies, “we expect this to have a significant societal impact.
“I am doing the research that I am interested in, but this is the research that companies are interested in as well,” he says. “And if it’s successful, it would come from us. It would come from Clark.”

“There’s this level of excitement that Julio brings to the laboratory setting — he makes it feel very alive.”
—katelynn humphrey ’25










Search
RECENT PRESS RELEASES
Related Post

