Characterization of terpene extract from Cannabis sativa flower and evaluation of its anti
July 19, 2025
Abstract
Inhibition of melanogenesis is of major interest in the cosmetic industry. The mechanism underlying melanogenesis includes regulating tyrosinase by activating various signaling pathways, such as protein kinase A and C pathways. Natural material-based agents have recently gained attention as alternative medicines to suppress melanogenic pathways. Cannabis sativa contains many bioactive compounds that are widely used in traditional herbal medicines. The aim of this study was to identify and assess the anti-melanogenic effect of terpene extract from Cannabis sativa flower (TCF) to explore novel depigmenting agents. Gas chromatography-mass spectrometry revealed that TCF contains five monoterpenes, eleven sesquiterpenes, and a diterpene. MTT and melanin content assays demonstrated that TCF significantly decreased the melanin content per live cell in a dose-dependent manner. Tyrosinase inhibition assay showed that TCF exhibited little inhibitory effect on tyrosinase at an equal dose, which reduced the melanin content. However, western blot analysis revealed that TCF downregulated microphthalmia-associated transcription factor (MITF), and tyrosinase-related proteins TRP1, and TRP2 expression by suppressing PKC, p38, and ERK/STAT3, while upregulated Akt pathway in melan-a. These results highlight the potential application of TCF as a natural whitening agent in the cosmetics industry.
Introduction
Pigmentation in human skin is caused by melanin synthesized by melanocytes. Melanin protects the skin from extrinsic factors, such as ultraviolet rays and pollutants. Various signaling pathways, such as the protein kinase C (PKC) signaling pathway can induce melanogenesis, and ultimately activating the master regulator microphthalmia-associated transcription factor (MITF)1,2. MITF, once activated, regulates the expression of tyrosinase, and tyrosinase-related proteins TRP1 and TRP2, which catalyze the conversion of tyrosine to melanin3. Tyrosinase initiates melanin synthesis by sequentially oxidizing tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) and dopaquinone. Dopaquinone is then converted to dopachrome by a chemical reaction, which in turn becomes melanin catalyzed by tyrosinase, TRP1, and TRP24. Thus, the suppression of MITF, tyrosinase, TRP1, and TRP2 is considered a major target for developing anti-melanogenesis agents and therapies5. Many whitening compounds (e.g., kojic acid, arbutin, and hydroquinone) have been discovered; however, they are difficult to commercialize because of their side effects such as contact dermatitis6. Therefore, exploring substitute materials to overcome these shortcomings is imperative, and natural material-based antimelanogenetic compounds may offer an alternative.
Cannabis sativa L. (C. sativa), also known as hemp or marijuana, has been used since ancient times as a source of medicine and textile fibers7. Its flower, in particular, contains numerous phytochemicals including cannabinoids, terpenes, Δ9-tetrahydrocannabinols (THC), and cannabidiols (CBDs), thus used in medicine for its medicinal properties8. However, the use of C. sativa flowers is limited as THC is one of the major psychoactive compounds. This plant can be legally used only when it contains 0.2–0.3% THC9 and only seeds in all parts of C. sativa have high industrial value because their THC content is below 0.3%10.
Terpenes are the major constituents of essential oils and are found in several plants, including C. sativa. Apart from CBDs (125 compounds), terpenes (120 compounds) constitute the largest class of C. sativa components11 and are especially abundant in flowers12. Terpenes, which contain the carbon backbone of isoprene, are produced in the plant cell cytoplasm via the mevalonic acid pathway13. Their primary function is to defend against predators and pathogens14and their bioactive properties include antibacterial, anti-inflammatory, and antioxidant15. Despite possessing various bioactivities, only a few studies have evaluated the effect of these terpenes on melanogenesis.
The present investigation aimed to identify the constituents of TCF and determine their anti-melanogenetic effect on melanocytes to identify its potential applicability as a whitening agent in the cosmetic industry.
Materials and methods
Plant materials and terpene extract Preparation from Cannabis sativa flower (TCF)
Cannabis sativa flower was collected from Andong-si, Gyeongsangbuk-do, Republic of Korea, in February 2024, and provided by Sangsang Farm. The plant species were identified by Dr. Buyng Su Hwang in Nakdonggang National Institute of Biological Resources, Republic of Korea. A voucher specimen (MCO-NP-D0004) has been deposited at the Library of Natural Products Research Institute, Korea Institute of Science and Technology. TCF (321.06 g, dry weight) was extracted using EtOH at room temperature. The EtOH extracts were evaporated in vacuo to obtain a dark brown residue, which was then partitioned using gradient Si gel column chromatography with ethyl acetate and hexane. The solution was then concentrated in vacuo to obtain a white residue (249.2 mg). The constituent analysis of TCF was performed using gas chromatography-mass spectrometry (GC-MS) according to the following conditions: Capillary column (60 m × 0.25 mm), chromatographic furnace temperature (60 °C, 2 min; 60–150 °C, 3 °C min− 1; 150–180 °C, 1 °C min− 1; 180–220 °C, 5 °C min− 1; 220 °C, 5 min), injection and detector temperature (25 °C), flow rate of carrier gas (1 mL min− 1), sample size (0.2 µL), and split ratio (100 : 1).
Melan-a cell culture
Melan-a cell line (Sigma Aldrich, St. Louis, MO, USA) was cultured in Roswell Park Memorial Institute (RPMI) 1640 media (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA), 1% penicillin/streptomycin (HyClone Laboratories), and 200 nM phorbol 12-myristate 13-acetate (PMA, Sigma Aldrich). A total of 1 × 105 cells were cultured in 60 mm dish plate and then incubated at 37 °C with 5% CO2.
Cell viability
Melan-a cells (4 × 104) were seeded in each well of 96-well plate with Roswell park memorial institute (RPMI) media containing 200 nM PMA and incubated for 24 h. Various doses of TCF (3.9–1,000 ppm) were applied to the cells for 72 h. The media was then removed, and RPMI media containing 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma Aldrich) was added to each well, followed by incubation for 1 h. Dimethyl sulfoxide was then added to the wells and the absorbance was measured at 560 nm by using microplate spectrophotometer (Bio-Tek Power Wave XS, Winooski, VT, USA).
Melanin content assay
Melan-a cells (4 × 104) were cultured in each well of a 96-well plate with RPMI media with 200 nM PMA and incubated for 24 h. TCF (3.9–31.3 ppm) were applied to the cells for 72 h. The media was then removed, and 6 N NaOH was added to the wells and incubated for 30 min at 85 oC. The absorbance was measured at 490 nm using the spectrophotometer. Melanin content per live cell was calculated using the following formula:
Melanin content per live cells (%) = (Melanin content (Abs at 490 nm)) / (Cell viability (Abs at 560 nm)) × 100.
Tyrosinase activity assay
A tyrosinase activity inhibition assay was conducted using L-DOPA as substrate to assess the inhibitory effect of TCF against tyrosinase activity. In a 96-well plate, the assay was performed in four reaction mixture groups; A, 132 µL sodium phosphate buffer (0.1 M, pH 6.8), 20 µL mushroom tyrosinase (1,500 U/mL), 20 µL TCF (3.9–1,000 ppm), and 88 µL L-DOPA; A’, Mushroom tyrosinase replaced with 20 µL sodium phosphate buffer in A; B, Sample replaced with 20 µL sodium phosphate buffer in A; B’, Mushroom tyrosinase replaced with 20 µL sodium phosphate buffer in B. The total volume of each group was 220 µL, and the final concentration of L-DOPA was 1.5 mM. Tyrosinase and reaction mixtures without tyrosinase were prepared followed by preheating for 10 min at 37 °C, respectively. The preheated tyrosinase was then applied to the reaction mixture after incubating for 20 min at 37 °C using a microplate reader with shaking for 1 s every 30 s. After 20 min of incubation, the absorbance was measured at 490 nm. The tyrosinase inhibition activity was calculated as follows:
Tyrosinase inhibition activity (%) = 100 – ((Abs A – Abs A’) / (Abs B – Abs B’)) × 100.
Western blotting
Melan-a cells (1.6 × 106) were cultured in 60 mm dish plates with RPMI containing 200 nM PMA for 72 h Selected concentrations of TCF (7.8, 15.6 and 31.3 ppm) were applied to the cells for 72 h. Cells were then harvested using a scraper (Sarstedt, Nümbrecht, Germany) and protein extraction reagent (iNtRON Biotechnology, Seoul, Korea) containing a phosphatase inhibitor cocktail and protease. Protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, IL, USA). Appropriate amounts of protein were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and subsequently transferred onto polyvinylidene fluoride membranes. Then, the membranes were sequentially blocked with 5% skim milk solution (dissolved in Tris-buffered saline containing 0.05% Tween 20) and probed with primary antibodies against p-PKC, MITF, TRP1, TRP2, phospho-Ca2+/calmodulin-dependent protein kinase II (p-CAMKII), CAMKII, phospho-p38 MAPK (p-p38), p38, phospho-extracellular signal-regulated kinase (p-ERK), ERK, phospho-signal transducer and activator of transcription 3 (p-STAT3), STAT3, phospho-protein kinase A (p-PKA), PKA, phospho-protein kinase B (p-Akt), Akt, and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at 4 °C for 15 h. The membranes were incubated with appropriate secondary horseradish peroxidase (HRP)-conjugated antibodies. Each protein band was detected and visualized using the iBright CL1000 system (Thermo Fisher Scientific). The antibodies used for western blotting are listed in Table 1.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 24.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences between means were analyzed using one-tailed analysis of variance (ANOVA), followed by Tukey’s post-hoc test. Statistical significance was set at p < 0.05.
Results
Chemical characterization of TCF
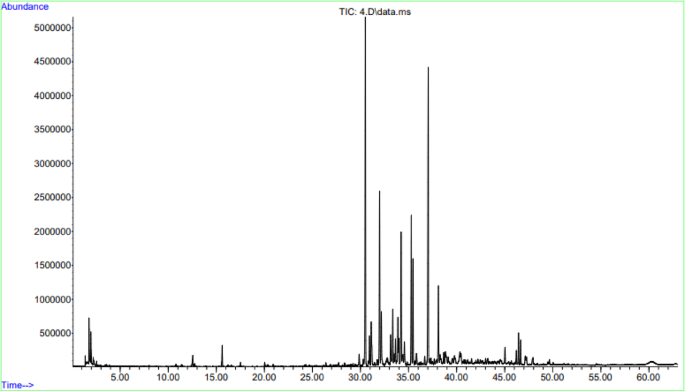
GC-MS was performed to determine the constituents of TCF. The results shown in Fig. 1; Table 2 indicate that TCF contains:
-
Five monoterpenes: cis-2,6-dimethyl-2,6-octadiene; p-cymene; D-limonene; benzene, 1-methyl-4-(1-methylethenyl); 4-acetyl-1-methylcyclohexene.
-
Eleven sesquiterpenes: caryophyllene; 1,5-cyclodecadiene; trans-α-bergamotene; benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl; naphthalene, decahydro-4a-methyl-1-methyl; naphthalene, 1,2,3,4,4a,5,6,8a-octahydro; β-bisabolene; selina-3,7(11)-diene; caryophyllene oxide; 3,7,11,15-tetramethylhexadec-2-ene; corymbolone.
-
One diterpene: neophytadiene.
TCF downregulates melanin content per live cells
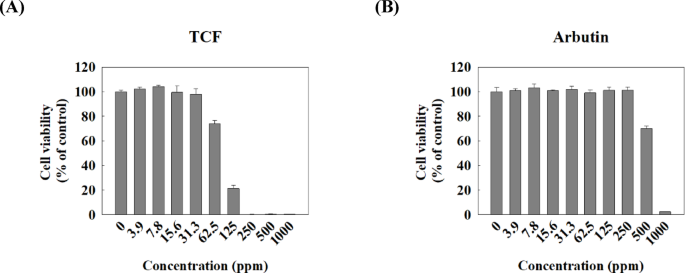
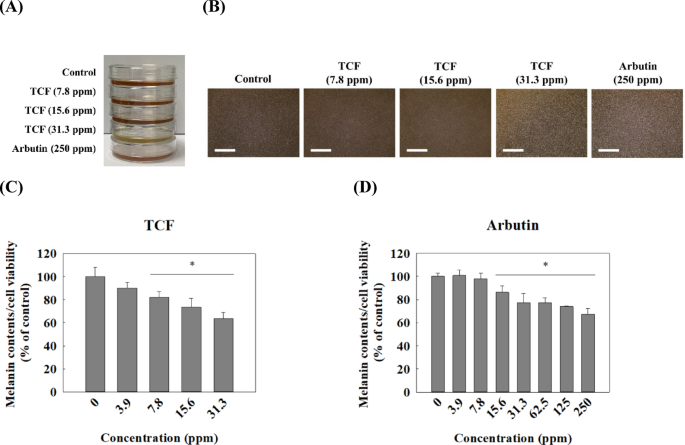
The MTT assay was conducted to determine the cytotoxicity of TCF in melan-a cells. TCF exhibited non-cytotoxicity at concentrations ranging from 0 to 31.3 ppm but 62.5 ppm or higher concentrations of terpenes showed dose-dependent cytotoxicity in melan-a cells (Fig. 2A). Arbutin, as positive control, demonstrated cytotoxicity in melan-a at concentrations of 500 µg/mL or more (Fig. 2B). As shown in Fig. 3A-B, the cell culture media treated with TCF showed dose-dependent brightness compared with the control group, and almost equal to that of the arbutin-treated group at 31.3 ppm of TCF-treated group. The melanin content in live melan-a cells was also calculated. The results revealed that the melanin content per live cell was 90.2%, 82.0%, 73.5%, and 63.6% at 3.9, 7.8, 15.6, and 31.3 ppm of TCF, respectively (Fig. 3C). Furthermore, 31.3 ppm of TCF reduced melanin content compared to 250 µg/mL of arbutin (Fig. 3C-D). Thus, we confirmed that melanin content in live cells, upon TCF treatment, decreased in a dose-dependent manner.
TCF downregulates melanin contents in melan-a cells. Photograph for (A) Extracellular melanin and (B) the melan-a cell line, and (C–D) melanin content in live melan-a cells post-72 h. Scale bar: 1000 μm. (C) TCF-treated group and (D) arbutin-treated group. Melanin contents in melan-a cell line was calculated using the following formula; Melanin contents/cell viability × 100 (%). Data are expressed as mean ± standard deviation of triplicate experiments. Statistical significance was analyzed using one-tailed one-way ANOVA, followed by Tukey’s post-hoc test (*p < 0.05).
Melanogenesis inhibitory mechanism of TCF
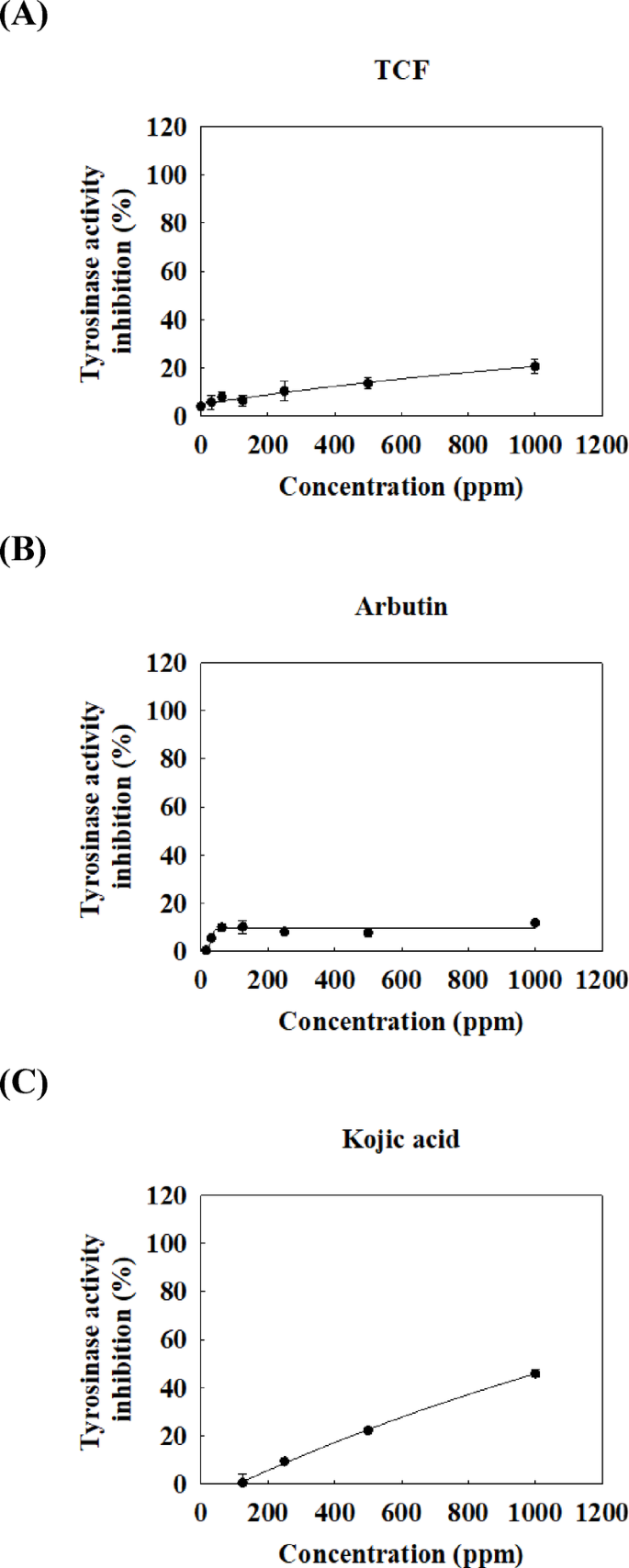
A tyrosinase inhibition assay was performed to determine whether the decrease in melanin content in melan-a cells was due to the inhibition of tyrosinase activity by TCF. In Fig. 4A, only 20% inhibition of tyrosinase activity was observed, even at a high terpene concentration (1000 ppm). Kojic acid and arbutin, which have strong tyrosinase inhibitory activities with L-dopa as the substrate16,17inhibited tyrosinase activity by approximately 50 and 10%, respectively (Fig. 4B-C).
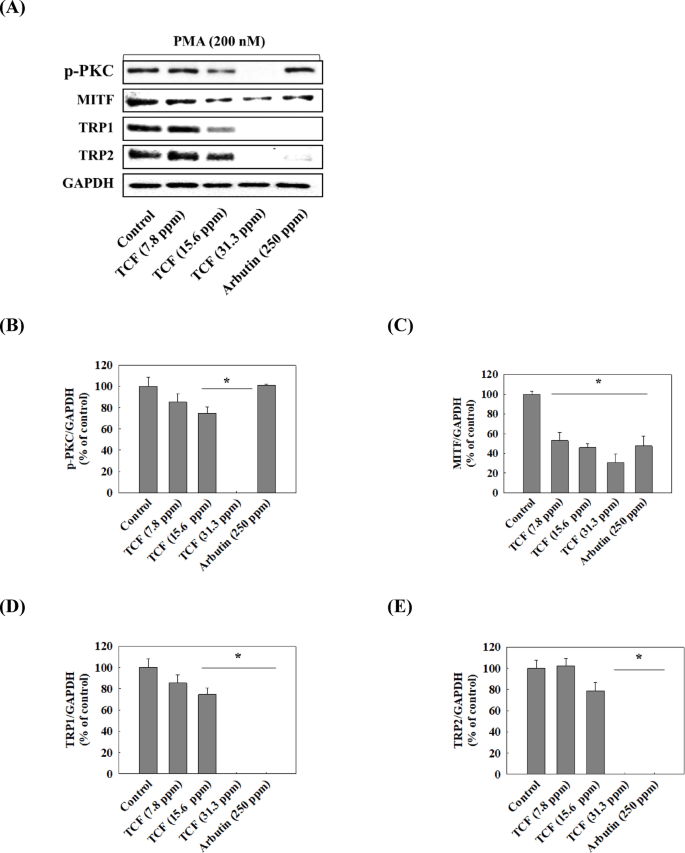
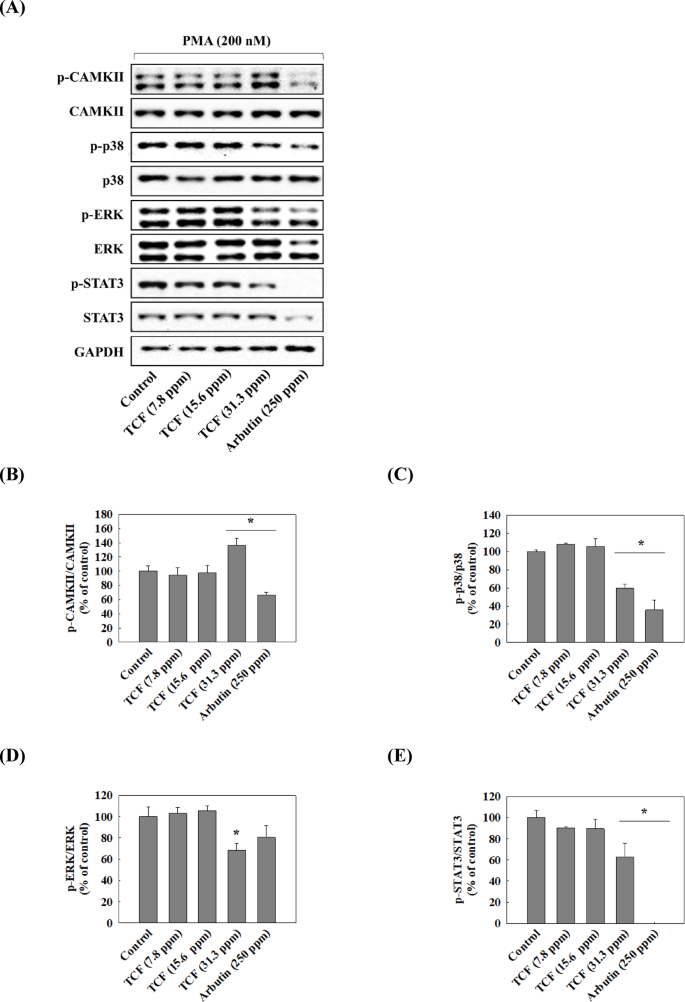
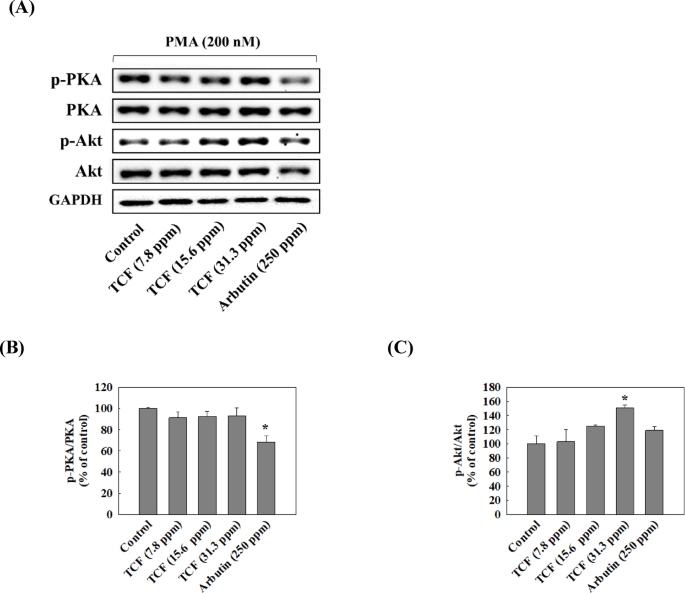
We attempted to confirm whether TCF downregulates the expression of TRP1 and TRP2 by suppressing MITF expression and to identify the upstream pathways regulating MITF. Figure 5 showed that TCF downregulated the phosphorylation of PKC and expression of MITF, TRP1, and TRP2 in a dose-dependent manner (7.8–31.3 ppm). Moreover, 31.3 ppm of TCF decreased MITF, TRP1, and TRP2 expression levels to that of 250 ppm of arbutin in melan-a cells. However, PKC phosphorylation remained unchanged in the arbutin-treated groups. As shown in Fig. 6 and 31.3 ppm of TCF increased CAMKII phosphorylation; however, the phosphorylation of p38, ERK, and STAT3 was downregulated. Arbutin regulates MITF expression by inhibiting PKA phosphorylation18but TCF had no effect on PKA (Fig. 7B). In contrast, TCF treatment upregulated Akt phosphorylation (Fig. 7C).
Effect of TCF on PKC pathway. (A) Representative western blots (n = 1 per lane) of p-PKC, MITF, TRP1, and TRP2. (B–E) Western blot densitometry results for (B) p-PKC, (C) MITF, (D) TRP1, and (E) TRP2. Statistical significance was analyzed using one-tailed one-way ANOVA, followed by Tukey’s post-hoc test (*p < 0.05). Original blots are presented in Supplementary Fig. 1.
Effect of TCF on CAMKII pathway. (A) Representative western blots (n = 1 per lane) of p-CAMKII, p-p38, p-ERK, and p-STAT3. (B–E) Western blot densitometry results for (B) p-CAMKII, (C) p-p38, (D) p-ERK, and (E) p-STAT3. Data are expressed as mean ± standard deviation of triplicate experiments. Statistical significance was analyzed using one-tailed one-way ANOVA, followed by Tukey’s post-hoc test (*p < 0.05). Original blots are presented in Supplementary Fig. 2. p-CAMKII, CAMKII, P-ERK, and ERK: the samples derive from the same experiment and that blots were processed in parallel.
Effect of TCF on Phosphorylation of PKA and Akt. (A) Representative western blots (n = 1 per lane) of p-PKA and p-Akt. (B and C) Western blot densitometry results for (B) p-PKA and (C) p-Akt. Data are expressed as mean ± standard deviation of triplicate experiments. Statistical significance was analyzed using one-tailed one-way ANOVA, followed by Tukey’s post-hoc test (*p < 0.05). Original blots are presented in Supplementary Fig. 3.
Discussion
Terpenes can be found in a wide variety of bacteria, fungi, algae, plants, and marine animals19,20. They all share isopentenyl pyrophosphate (IPP) in structure and are categorized based on the number of IPP units into hemiterpenes (one isoprene unit), monoterpenes (two isoprene units), sesquiterpenes (three isoprene units), diterpenes (four isoprene units), triterpenes (six isoprene units), tetraterpenes (eight isoprene units), and polyterpenes (more than eight isoprene units)21. Terpenes, especially sesquiterpenes, are low in number in plants and have low extraction efficiencies, making their industrial utilization difficult22. In the present study, we determined that TCF contains five monoterpenes, eleven sesquiterpenes, and one diterpene (Table 2). C. sativa flowers contain THC and CBD, which limits their use8,9. In this study, no THCs or CBDs were present in TCF (Table 2), demonstrating that C. sativa flowers are a good source of terpenes.
Terpenes are major constituents of plant resins and function as infochemicals, attractants, repellents, and defense against predators23,24. Terpenes possess various biological properties such as anticancer, antimalarial, neuroprotective, and anti-inflammatory activities25,26. However, few studies have reported the antimelanogenetic effects of terpenes27,28,29. In this study, we confirmed that TCF dose-dependently decreased melanin content (Fig. 3).
Most signaling pathways related to melanogenesis are initiated by different factors but ultimately converge on the activation of MITF in melanocytes. Activated MITF enhances the production of tyrosinase, TRP1, and TRP2, which participate in the conversion of tyrosine to melanin. Thus, many components with anti-tyrosinase activity or MITF inhibitory functions have been discovered and developed for use in the cosmetic industry. Moreover, most melanogenesis-inhibitory agents have only one strong target in melanogenesis, such as tyrosinase activity and MITF expression30. In this study, TCF has low inhibitory activity against tyrosinase. As a result, TCF is expected to exert an anti-melanogenic effect through a mechanism different from its tyrosinase inhibitory activity (Fig. 4).
MITF, which regulates the expression of tyrosinase and TRPs, may serve as a target for whitening agents. In melan-a cells, PMA induces the activation of PKC pathway, which further regulates MITF expression31,32. In this study, TCF inhibited expression of MITF, TRPs and suppressed PKC pathway (Fig. 5). CAMKII phosphorylation regulates the phosphorylation of p38 and ERK in melanogenesis33. Also, activated ERK/STAT3 and p38 pathways increase MITF expression34 while Akt inhibits it35. In this study, TCF upregulated the phosphorylation of CAMKII, but inhibited p38 and ERK/STAT pathways (Fig. 6) and activated the Akt pathway (Fig. 7). Therefore, TCF is expected to suppress MITF expression by inhibiting PKC, p38, and ERK/STAT3 pathways and activating Akt pathway.
In this study, we revealed that TCF contains five monoterpenes, eleven sesquiterpenes, and a diterpene. While most of them lack studies confirming their anti-melanogenic effect, a previous study revealed that some terpenes, such as loliolide, D-limonene, caryophyllene, and caryophyllene oxide, but not p-cymene, have anti-melanogenetic effects36,37. Therefore, we hypothesized that TCF has anti-melanogenetic effects owing to the presence of D-limonene, caryophyllene, and caryophyllene oxide, although the melanogenesis inhibitory effect of other terpenic compounds needs to be confirmed. In summary, this study is the first report to reveal the anti-melanogenic mechanism of TCF, indicating that C. sativa flowers have the potential for industrial application as a source of terpenes and whitening agents.
Conclusion
The current study unveiled that TCF contains three classes of terpenes, monoterpenes, sesquiterpenes, and diterpenes, and exhibits anti-melanogenetic effects in melan-a cells. The non-cytotoxic dose of TCF downregulated melanin content in melan-a cells, and this effect was greater than that of the positive control, arbutin. TCF demonstrated the ability to inhibit MITF, which regulates the expression of tyrosinase, TRP1, and TRP2, albeit lacking tyrosinase inhibitory activity. TCF regulated MITF by suppressing PKC, p38, and ERK/STAT3 pathways, and activating the Akt pathway. Further studies are needed to identify crucial terpenic compounds with anti-melanogenesis inhibitory activity, which would facilitate the use of C. sativa flowers in the cosmetics industry as whitening agents and sources of terpenes.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Info files).
References
-
Niu, C. & Aisa, H. A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 22 (8), 1303 (2017).
-
Kim, K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J. Ginseng Res. 39 (1), 1–6 (2015).
-
Tsang, T. F. et al. Wendy hsiao, W. L. Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/β-catenin signaling pathways. Phytomedicine 60, 153008 (2019).
-
El-Nashar, H. A. S., El-Din, M. I. G., Hritcu, L. & Eldahshan, O. A. Insights on the inhibitory power of flavonoids on tyrosinase activity: A survey from 2016 to 2021. Molecules 26 (24), 7546 (2021).
-
Kumari, S., Thng, S. T. G., Verma, N. K. & Gautam, H. K. Melanogenesis inhibitors. Acta Derm Venereol. 98 (10), 924–931 (2018).
-
Masub, N. & Khachemoune, A. Cosmetic skin lightening use and side effects. J. Dermatol. Treat. 33 (3), 1287–1292 (2022).
-
Peters, H. & Nahas, G. G. A brief history of four millennia (BC 2000—AD. in Marihuana and medicine (ed. Nahas, G. G., Sutin, K. M., Harvey, D., Agurell, S., Pace, N. & Cancro, R.) 3–7 (Humana Press, 1999). (1974).
-
Pellati, F. et al. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L.(hemp). Molecules 23 (10), 2639 (2018).
-
Hartsel, J. A., Eades, J., Hickory, B. & Makriyannis, A. Chapter 53 – Cannabis sativa and hemp in Nutraceuticals (ed. Gupta, R. C.) 735–754 (Academic Press, 2016).
-
Rehman, M. et al. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut Res. Int. 28 (38), 52832–52843 (2021).
-
Radwan, M. M., Chandra, S., Gul, S. & ElSohly, M. A. Cannabinoids, phenolics, terpenes and alkaloids of Cannabis. Molecules 26 (9), 2774 (2021).
-
Roell, M. S. Terpenes in cannabis: Solving the puzzle of how to predict taste and smell. Plant. Physiol. 184 (1), 8–9 (2020).
-
Stephane, F. F. Y. & Jules, B. K. IntechOpen; J. Terpenoids as important bioactive constituents of essential oils in essential oils-bioactive compounds, new perspectives and applications (ed. De Oliveira, M. S., Silva, S. & Da Costa, W. A.) 1–15 (2020).
-
Gershenzon, J. & Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3 (7), 408–414 (2007).
-
Masyita, A. et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X. 13, 100217 (2022).
-
Phasha, V. et al. Review on the use of Kojic acid—A skin-lightening ingredient. Cosmetics 9 (3), 64 (2022).
-
Boo, Y. C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 10 (7), 1129 (2021).
-
Choi, M. H., Yang, S. H., Kim, N. D. & Shin, H. J. Nomilin from Yuzu seed has in vitro antioxidant activity and downregulates melanogenesis in B16F10 melanoma cells through the PKA/CREB signaling pathway. Antioxidants 11 (9), 1636 (2022).
-
Schulz, G. E., Schirmer, R. H., Sachsenheimer, W. & Pai, E. F. The structure of the flavoenzyme glutathione reductase. Nature 273 (5658), 120–124 (1978).
-
Dudareva, N. et al. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. U. S. A. 102 (3), 933–938 (2005).
-
Ruzicka, L. The isoprene rule and the biogenesis of terpenic compounds. Experientia 9 (10), 357–367 (1953).
-
Mai, J., Li, W., Ledesma-Amaro, R. & Ji, X. J. Engineering plant sesquiterpene synthesis into yeasts: A review. J. Agric. Food Chem. 69 (33), 9498–9510 (2021).
-
Degenhardt, J., Gershenzon, J., Baldwin, I. T. & Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 14 (2), 169–176 (2003).
-
Junker, R. R., Gershenzon, J. & Unsicker, S. B. Floral odor bouquet loses its ant repellent properties after Inhibition of terpene biosynthesis. J. Chem. Ecol. 37 (12), 1323–1331 (2011).
-
Cho, K. S. et al. Terpenes from forests and human health. Toxicol. Res. 33 (22), 97–106 (2017).
-
Maimone, T. J. & Baran, P. S. Modern synthetic efforts toward biologically active terpenes. Nat. Chem. Biol. 3 (7), 396–407 (2007).
-
Yang, C. H. et al. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 37 (5), 550–554 (2015).
-
Ko, G. A. & Cho, S. K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem. Biol. Interact. 286, 132–140 (2018).
-
Sharma, C. et al. Polypharmacological properties and therapeutic potential of β-Caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 22 (21), 3237–3264 (2016).
-
Pillaiyar, T., Manickam, M. & Jung, S. H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today. 22 (2), 282–298 (2017).
-
Kauser, S., Schallreuter, K. U., Thody, A. J., Tobin, D. J. & Gummer, C. Regulation of human epidermal melanocyte biology by β-Endorphin. J. Investig. Dermatol. 120 (6), 1073–1080 (2003).
-
Nishizuka, Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258 (5082), 607–614 (1992).
-
Regazzetti, C. et al. Melanocytes sense blue light and regulate pigmentation through opsin-3. J. Investig. Dermatol. 138 (1), 171–178 (2018).
-
Dong, L. et al. FGF2 regulates melanocytes viability through the STAT3-transactivated PAX3 transcription. Cell. Death Differ. 19 (4), 616–622 (2012).
-
Choi, H., Yoon, J. H., Youn, K. & Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, mapks, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 147, 112651 (2022).
-
Torrens, F. & Castellano, G. Oxidative stress-protective/anti-melanogenic effects of loliolide in natural products pharmacology and phytochemicals for health care (ed. Mahapatra, D. K., Aguilar, C. N. & Haghi, A. K.) 107–115 (Apple Academic Press, 2021).
-
Kumar, K. J. S. et al. Essential oils of Alpinia nantoensis retard forskolin-induced melanogenesis via ERK1/2-mediated proteasomal degradation of MITF. Plants 9 (12), 1672 (2020).
Funding
This work was supported by the following grants and programs: the Technological Innovation R&D Program (no. P0023712) funded by the Ministry of SMEs and Startups (Republic of Korea); a grant (RS-2024-00332024) from the Ministry of Food and Drug Safety (Republic of Korea); the Ministry of Science and ICT (Republic of Korea) support program (2021-DD-UP-0379); and an intramural research grant (Development of Data Utilization Technology for Natural Product Research, 2E33521) from the Korea Institute of Science and Technology (KIST). Finally, the data were deposited in the KIST Dashboard.
Author information
Authors and Affiliations
Contributions
J-.W.K.: Data curation, formal analysis, investigation, methodology, validation, visualization, original draft, and writing. Y.H.: Resources, formal analysis, investigation, methodology. B.D.: Formal analysis, software. P-.S.C.: Supervision, review, and editing. T.K.: Methodology, review, funding and editing. J.H: Review, and editing. J-.C.K.: Project administration, supervision, review, and editing. All the authors have read and agreed to the published version of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, JW., Han, Y., Dorjsembe, B. et al. Characterization of terpene extract from Cannabis sativa flower and evaluation of its anti-melanogenetic effect in melan-a cells.
Sci Rep 15, 26231 (2025). https://doi.org/10.1038/s41598-025-10820-6
-
Received: 16 May 2025
-
Accepted: 07 July 2025
-
Published: 19 July 2025
-
DOI: https://doi.org/10.1038/s41598-025-10820-6
Keywords
Search
RECENT PRESS RELEASES
Related Post