NJ-CRC Releases New Cannabis Testing Rules to Increase Quality
March 3, 2025
The NJ Cannabis Regulatory Commission (NJ-CRC) recently released the full updated cannabis lab testing rules they announced at their last meeting.
Cannabis testing laboratories have until Wednesday, March 19th, to implement sampling changes and until midnight on Friday, May 23rd for “initial and stability testing changes.”
While the rules are very complicated and very technical, they are very important in establishing high product standards in the New Jersey cannabis market.
They should address concerns about quality, like the amount of mold found in some flower.
It will be interesting if any companies are penalized and made an example of after the deadlines.
The new standards include tests for foreign matter, pesticides, microbes, mycotoxins, and heavy metals.
Notably, the rules allow remediation. That is when moldy weed gets blasted with radiation waves you wouldn’t want to be exposed to unless you want to be in a Marvel movie.
The new rules specify a maximum lot size for testing cannabis concentrates, vaporized formulations, and ingestible, transmucosal (through the mouth), and dermal (skin) cannabis-infused products.
The guidance also further standardizes lab testing methods. Those methods include requiring culture-based testing for total bacteria and mold counts, testing for harmful pathogens, and a temperature range for sample drying.
In 2021, the NJ-CRC quickly adopted Maryland’s medical marijuana testing rules. This was when there was great pressure for action once they were established after so many delays which built upon each other.
Once a testing laboratory begins sample collection, the medical Alternative Treatment Center (ATC) or cannabis business is supposed to use the same lab for all the testing.
So, “cannabis business or testing laboratory requesting changes between initial sampling and retention sampling to the laboratory, technology, or protocols shall submit a request for approval to the Commission.”
“Where one of the parties of the testing contract breaks the terms of the agreement, the testing laboratory and the cannabis business or ATC shall notify the Commission within 72 hours of such breach,” they said.
One of the big things the NJ-CRC wants to prevent is lab shopping, whereby a grower or manufacturer takes their product to different labs to get different test results.
Generally, they want to seem like they have the strongest weed with the most THC, which most know gets you high.
Most consumers do not know enough about terpenes and cannabinoids to take them into account when looking for a quality product. It is most likely due to federal marijuana prohibition.
So weed with the most THC is often selling for the most since that’s what adult-use recreational cannabis consumers seem to want.
Plus, they don’t want to seem like they have moldy weed.
Testing scientists are now supposed to do a visual inspection of cannabis and cannabis products to screen for foreign material.
“If visible foreign material such as sand, soil, cinders, dirt, mold or mildew exceeds 10% of the total sample area, the sample shall not meet specifications,” they said.
Also, “if visible foreign material exceeds one insect fragment, one hair, or one count of mammalian excreta (urine and feces) per 3.0g, the sample shall not meet specifications.”
Inspections include looking at the interior and exterior of the dried flower and plant material. For a cannabis product, inspection includes the exterior of the product.
The testing laboratory shall test cannabis and cannabis products for microbial contamination in Colony Forming Units (CFU/g), the rules say.
So, they want to eliminate any weed with microbes like bacteria.
They want weed tested for pesticides, fungicides, pathogens, salmonella, E. coli plant growth regulators, listeria monocytogenes, mycotoxin contamination, among other things, to ensure only quality cannabis is for sale.
The rules say that “the testing laboratory shall test cannabis concentrates and cannabis-infused products for residual solvents and other manufacturing residue, including inhalable products.”
They also want to test for propane, chloroform, butane, and ethanol to ensure those oil-based chemicals are not in any quality New Jersey cannabis products.
Different types of products have different limits outlined in the rules.
New Testing Standards for Legal NJ Weed with Bad Heavy Metals
The NJ-CRC wants to ensure no corners are cut and harmful chemicals or metals are found in the products they regulate. So now they want labs to test for arsenic, cadmium, chromium, lead, and mercury, among other heavy metals.
If an initial sample does not meet the specifications of this Testing Guidance, the testing laboratory shall follow its standard operating procedure to confirm or refute the original results.
Notably, labs are supposed to test cannabis vape oils for Vitamin E Acetate.
The NJ-CRC wants 30 samples taken for every 33.7 pounds of cannabis.
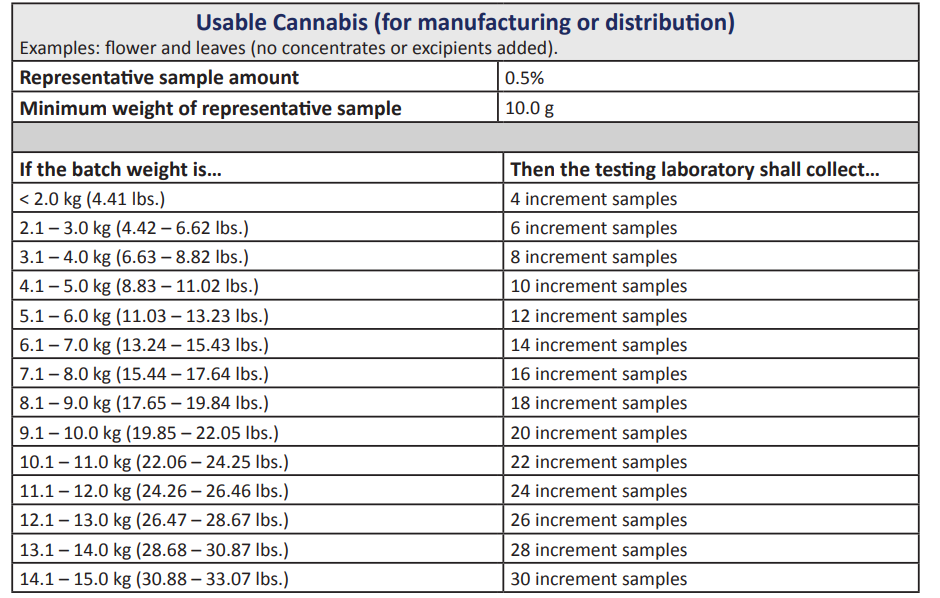
It seems like they want every basic increment of cannabis products tested.
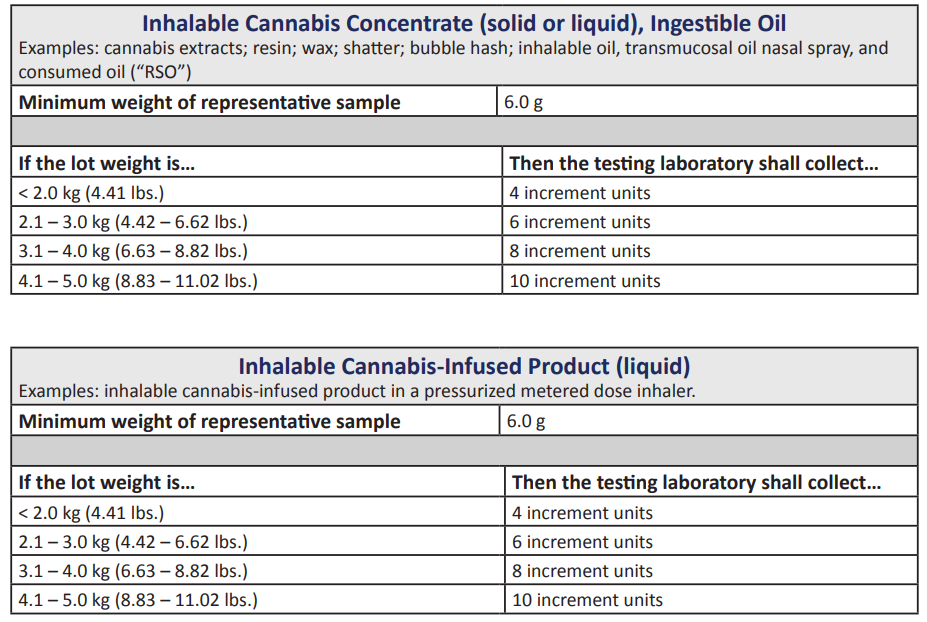
The rules establish high standards to prevent contamination.
So, they want a lot of samples for every type of product sold in the New Jersey cannabis market. The NJ-CRC wants to be sure that the samples are representative of the cannabis batches.
“Samples must be removed from the top, middle, and bottom of containers, with the top sample being taken from a depth of not less than 10 centimeters,” the rules say.
Where there are fewer than 11 containers in the batch or lot, the lab technician should get at least one sample or unit from each container.
Some cannabis advocates have spoken passionately during NJ-CRC meetings about the need to decrease testing batch sizes.
Heady NJ has heard stories that often, very low-quality cannabis is used to make distillate oil. It then goes into gummies and vape cartridges or carts.
Notably the testing rules allow for remediation, a form of radiation to kill mold and similar things.
“If retesting confirms that the sample does not meet specifications, the ATC/cannabis business may, upon notice to the Commission, remediate the batch or lot from which the failed sample was taken,” the rules say.
The remediated batch or lot shall be subject to a subsequent test of a new representative sample.
The testing laboratory shall retain the remains of the initial sample for 30 days after analysis is completed.
At such time, the testing laboratory shall destroy the sample or render it unrecoverable and unrecognizable.
The NJ-CRC wants to increase the standards of cannabis and testing.
So “a testing laboratory employee shall collect a representative initial sample and a representative retention sample from each batch of unusable cannabis for manufacturing or usable cannabis from a cannabis cultivator and from each lot of cannabis products from a cannabis manufacturer according to a statistically valid sampling method.
Notably, a “cannabis business employee shall be physically present to observe the testing laboratory employee collect any sample.”
The cannabis business employee is not supposed to touch the cannabis or the sampling equipment “while the testing laboratory employee is collecting the samples,” they said.
“By conducting stability testing on a product batch at various time intervals, manufacturers effectively assess cannabinoid degradation and the potential rise in contaminants, such as microbes, over time,” the rules explained. “This data is crucial for establishing the product’s shelf life and determining appropriate expiration dates, ensuring consumer safety and product efficacy.”
In addition, they want all the products handled carefully including a lot of labeling.
“Testing laboratories must conduct stability testing on the collected retention samples to ensure product potency and purity and support or debunk the listed expiration date of the batch or lot,” the rules say. “The testing laboratory shall provide the sample’s written report and its data … to the cannabis business or ATC and shall submit identical data to the inventory control system.”
Notable “cannabis that will be manufactured into a cannabis product shall be tested… even where a vertically integrated cannabis cultivator is transferring it to a co-located cannabis manufacturer,” the rules say.
Cannabis cultivators are supposed to ensure quality cannabis does not have any mold, rot, or disease through a visual inspection.
According to the rules, labels of cannabis items should include serving size, the total number of servings contained, the cannabinoids, and the terpene profile.
The NJ-CRC rules want the weed kept very secure during the process, especially during transportation.
Cannabis for manufacturing retention samples are supposed to be stored by the cultivator or manufacturer for possible recall testing.
A cannabis business needs to store samples of cannabis for manufacturing for 60 days.
The NJ-CRC does not want a lab testing cannabis provided by a consumer, including underground legacy cannabis.
“A licensed testing laboratory shall not acquire or receive personal use usable cannabis or cannabis products, except from a cannabis business… and shall not distribute, sell, or dispense cannabis or cannabis products.”
“The testing laboratory shall test usable cannabis for distribution, cannabis concentrates, and cannabis-infused products to determine the chemical composition and potency of individual cannabinoids,” the new rules say.
The testing laboratory is not required to perform this test on cannabis that will be manufactured by a cannabis business/ATC into a product.
But may perform it upon request of the cannabis business/ATC.
The test shall include:
• delta-9-Tetrahydrocannabinolic Acid (THCA);
• delta-9-Tetrahydrocannabinol (THC);
• Cannabidiolic Acid (CBDA);
• Cannabidiol (CBD);
• Cannabigerolic Acid (CBGA);
• Cannabigerol (CBG); and
• Cannabinol (CBN);
The test may include other cannabinoids, such as:
• delta-8-Tetrahydrocannabinol (d8-THC);
• delta-9-Tetrahydrocannabivarinic acid (THCVA);
• delta-9-Tetrahydrocannabivarin (THCV);
• Cannabidivarinic Acid (CBDVA);
• Cannabidivarin (CBDV);
• Cannabichromenic Acid (CBCA); or
• Cannabichromene (CBC);
The rules say the testing laboratory shall test usable cannabis for distribution, cannabis concentrates, and cannabis-infused products formulated or labeled to contain terpenes to determine the chemical composition and potency of the individual terpenes.
The testing laboratory is not required to perform this test. But it may perform it upon request of the cannabis business/ATC.
The test shall include the following terpenes:
• alpha-Pinene;
• beta-Caryophyllene;
• beta-Myrcene;
• d-Limonene;
• Ocimene;
• Terpinolene;
• alpha-Humulene;
• beta-Pinene
• Linalool.
“For cannabis concentrate intended for inhalation or vaporized formulation, to meet specifications, the sample shall not exceed a level of terpenes that is 10% of the product by volume,” the rules say.
“Upon request of an ATC, cannabis business, patient, caregiver, consumer, or the Commission, the testing laboratory shall test vaporized formulation for the presence of other additives, cutting agents, and artificial flavorings known to be harmful,” the rules say.
But “such testing methods are not required to be included in a testing laboratory’s scope of accreditation.”
“After the foreign material screening, the testing laboratory shall test usable cannabis for distribution, solid ingestible cannabis-infused products, and solid transmucosal cannabis-infused products for water activity,” they said.
Too much water is likely to lead to mold and other problems.
The testing laboratory is not required to perform this test but may perform it upon request of the cannabis business.
“The testing laboratory shall test usable cannabis for distribution for moisture content percentage,” the rules say.
When drying a representative sample of usable cannabis in preparation for testing, a testing laboratory shall not exceed 105 degrees Celsius.
The cannabinoid profile shall reflect for the consumer the total THC, CBD, and CBG in the product.
Total THC in a cannabis product can include THC along with the traditional Delta-9-THC. Total CBD similarly includes CBDA.
The cannabinoid profile may reflect the total CBG, CBN, CBC, THCV, CBDV, or other cannabinoids.
The Commission encourages product labels that include the total CBG and CBN. It’s important for consumers and patients to know.
One of the things mentioned at the last NJ-CRC meeting was the establishment of a state-run reference lab to check the licensed labs. However it was not in the new testing rules
Search
RECENT PRESS RELEASES
Related Post






