NEOSTEM IS NOW CALADRIUS, INC (CLBS: NasdaqCM)
Caladrius (formerly NeoStem) is among the first of a new breed of immunotherapy companies with proven expertise and unique experience in cell process optimization, development, and manufacturing
Caladrius is pursuing the preservation and enhancement of human health globally through the development of cell based therapeutics that prevent, treat or cure disease.Our business includes the development of novel proprietary cell therapy products, as well as a revenue-generating contract development and manufacturing service business that we leverage for the development of our therapeutics while providing service to other companies in the cell therapy industry. ![]()
The company strives to be more than just producers of cell therapies and looks to develop and market therapies and platform technologies that will have significant and meaningful impact in the biotechnology sector. While aggressively pushing forward this development, NeoStem pledges to never lose sight of its responsibilities to the health and wellness of the public.
Overview
- Integrated Biotechnology Company with as strong pipeline based on multiple platform technologies that include Phase 2 and 3 assets and a revenue generating contract development and manufacturing business
- Five (5) issued patents, seventeen (17) matter and method patterns and forty-four (44) pending patents
- Exclusive rights to twenty-three (23) issued and nine (9) pending patents
in-licensed from the University of Louisville to the world-wide patent rights for the isolation, purification and therapeutic use of very small embryo-like (VSEL) stem cells - Headquarters in New York
- GMP compliant facilities in Allendale-NJ, Mountain View-CA and
Irvine-CA - 168 employees as of September 30, 2014
- Executed six sector specific acquisitions
- NASDAQ CM: CLBS
- Market Cap $144 Mill
- $32.8 Mill in cash and marketable securities as of September 30, 2014
VALUE PROPOSITION
A Late Stage Clinical Pipeline and Revenue Generating Service Business in Cell Therapy
Target Indications
- Stage IV and recurrent Stage II melanoma
- Acute myocardial infarction
- Steroid resistant Asthma
Research and Discovery
CLBS is combining state-of-the-art science with the body’s intricate biologic systems, and are working to leverage our bodies’ development of cell capabilities with complex and specific functions.CLBS believes that each study we perform expands the foundations of cell therapy and biologic science and believe that our research and discovery will have meaningful and lasting influence. CLBS pledges to use its experience and knowledge to advance science, research and development.
Patients
CLBS conducts all of our work in order to ultimately benefit the patients who put their trust and hope in our hands. CLBS will continue to pursue therapies in areas of significant unmet medical need so that we can have the greatest impact on them. CLBS knows it won’t be easy, but we are committed to striving to successfully guide its product candidates through the development process in order that they may ultimately reach and serve the patients.
Education
The cell therapy industry needs credible sources disseminating information. We pledge to be that credible source, promoting ethical science and ethical business, and playing an educational role in bringing cell therapy to the public which includes dispelling controversy, decreasing confusion and increasing awareness of cellular therapies and their potential to heal the body and fight disease.
Development Highlights
NeoStem is a leader in the emerging cellular therapy industry, pursuing the preservation and enhancement of human health globally through the development of cell based therapeutics that prevent, treat or cure disease by repairing and replacing damaged or aged tissue, cells and organs and restoring their normal function.
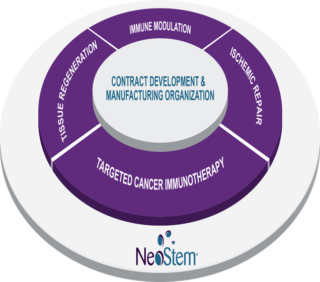
The business includes the development of novel proprietary cell therapy products as well as a revenue-generating contract development and manufacturing service business. This combination has created an organization with unique capabilities for cost effective in-house product development and immediate revenue and cash flow generation.
The CLBS mission is to transform the future of medicine with innovative cell based therapies while providing development and manufacturing services that drive the industry forward. CLBS is committed to showing the world the path to better medicine. CLBS’s goal is to reduce a lifetime dependency on pills to a single dose of cells and help society reduce the burden of an unsustainable healthcare system. The CLBS vision is a world where chronic disease is a problem of the past and patients have the freedom to enjoy a healthier span of life. CLBS truly believe that cell therapies will be better medicine.
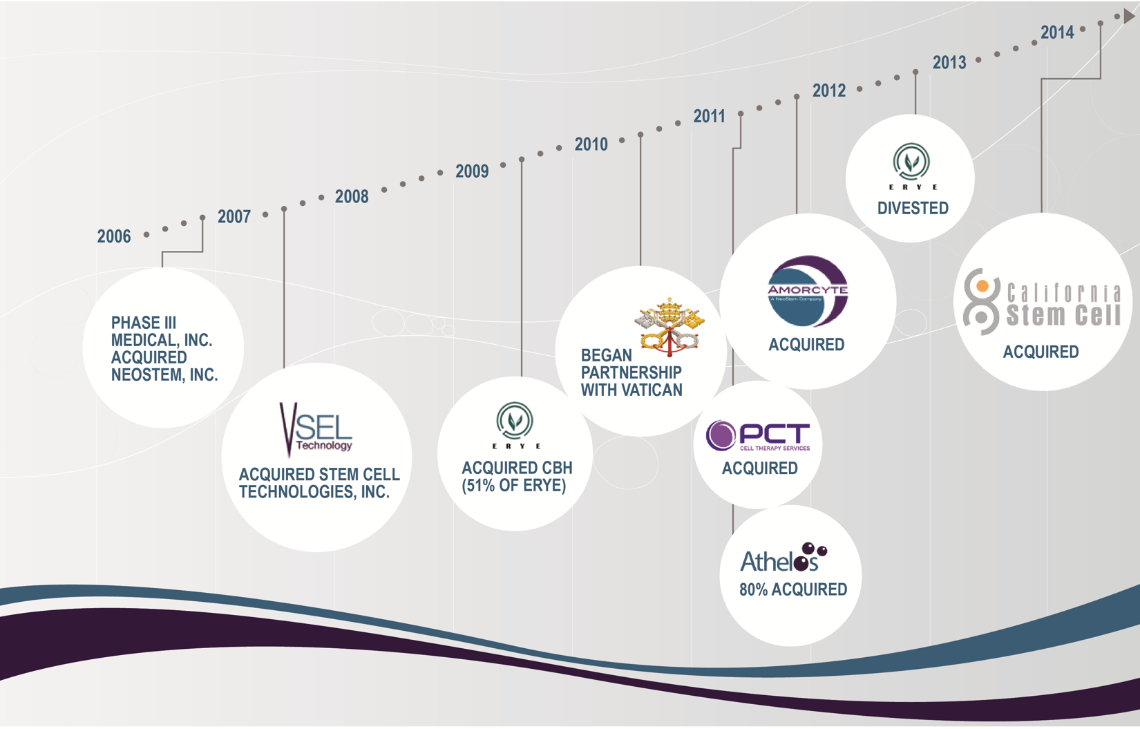
Caladrius Biosciences is leading the paradigm shift in modern medicine towards cell therapy – a shift away from treating disease with drugs and toward treating disease with the body’s own cells. Since 2006, Caladrius Biosciences has been led by physician and business executive Dr. Robin L. Smith and now Dr. David J. Mazzo, Phd. Dr. Mazzo and his team saw the potential to take part in this shift by developing the cell therapies that will potentially change the way that we treat illness and chronic disease. Just as the cell therapy industry has grown and developed over the past decade, CBLS too has transformed itself through a series of strategic mergers and acquisitions. First focusing on adult stem cells, and then expanding into therapeutics that work with other cell sources, CBLS has seized upon the opportunity to be more than just a participant in the emerging cell therapy industry, instead choosing to be a driving force for growth and advancement in cell therapy research, development and manufacturing.
For more than 15 years, PCT (now a CBLS subsidiary) has been providing market-leading contract development and manufacturing services to a wide range of clients in the regenerative medicine industry. With CBLS’s acquisition of PCT in 2011, CBLS gained access for its products to PCT’s world-class development expertise and manufacturing capabilities, as well as to a unique platform offering experience with multiple cell types. Also in 2011, CBLS acquired the technologies for its Ischemic Repair Program and Immune Modulation Program, expanding the Company’s development pipeline. In 2014 CBLS’s pipeline expanded further with its acquisition of California Stem Cell, creating the Company’s Targeted Cancer Immunotherapy Program and beginning CBLS’s investigation into cancer therapeutics. Along the way, the Company has sustained its successful growth by investing in a management team of seasoned industry executives and adding clinical programs for new indications.
A Portfolio of Cell Therapy Products In Development That Leverages The Body’s Natural Ability To Heal And Fight Disease
Five (5) issued patents and 35 pending patents in the US and OUS with covergae including:
- Stem Cell Growth, Generation and Use
- Antigen-presenting cancer vaccines
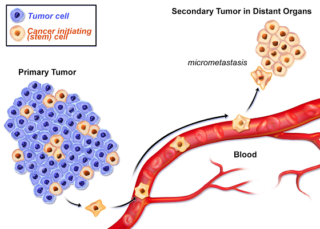
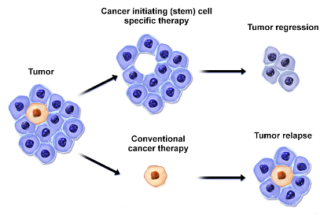
- Individualized high-purity carcinoma indicating stem cells
- Rapid methods to produce high purity cancer initiating (stem) cells)
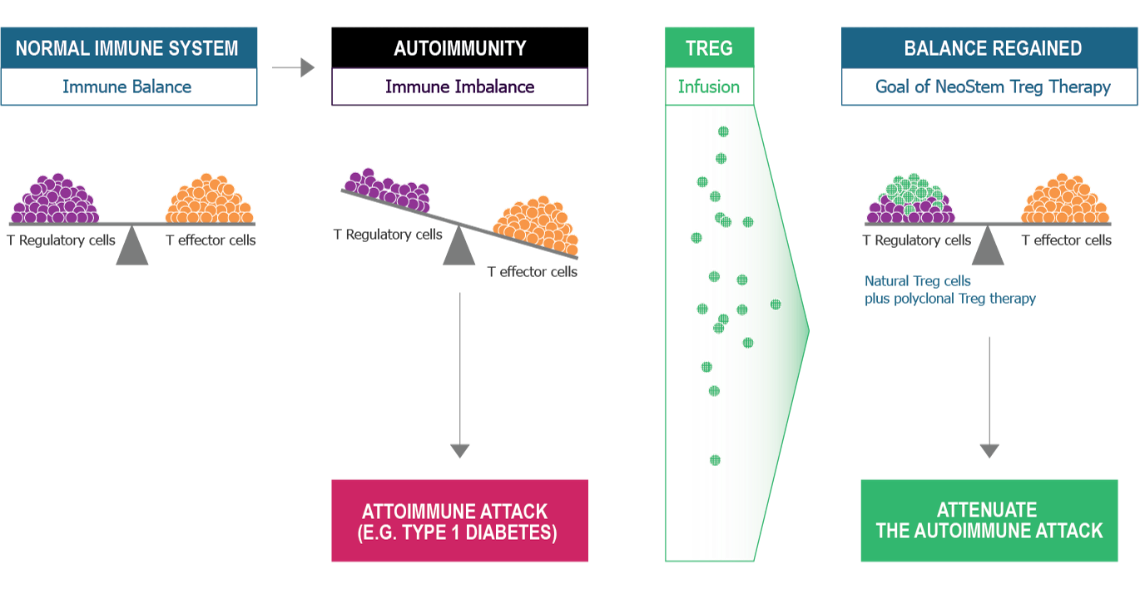
Using T Regulatory Cell Technology. Exclusive Rights to twenty-three (23) issued patents and nine (9) pending patents covering isolation, activation, expansion and methods of treating or preventing certain conditions and/or diseases using Tregs in the US and International markets. This includes the composition of matter patents and method patents.
TREG Therapy represents a novel approach for restoring Immune Balance by enhancing T-regulatory cell number and function
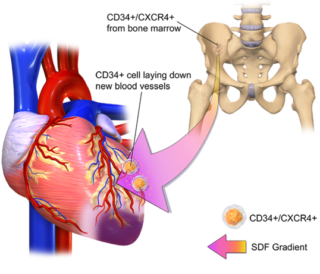
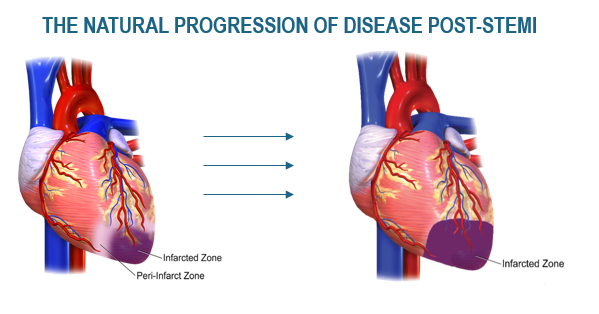
Broad and growing patent portfolio supports cardiac conditions and a broad range of other conditions caused by underlying ischemia, including the composition of matter and method patents. Ishemia occors when the supply of oxinated blood is restricted. The program seeks to reverse this restriction thorugh the developemnt and formation of new blood vessels. CD34/CXCR4 expressing cells have been shown to be capable of inducing the development and formation of new blood vessels and thus preventing heart cell death. The same natural repair mechanism applies to multiple areas of vascular insiffcinecy such as:
- Acute myocardial infarction
- Traumatic brain injury
- Chronic heart failure
- Crticial limb ischemia
First Target Indication
- 240,000 STEMI patients per year in the US
- ST segment elevation MI (STEMI) patients with high risk
- Average Age of 66
- $37 Billion hospital cost per year
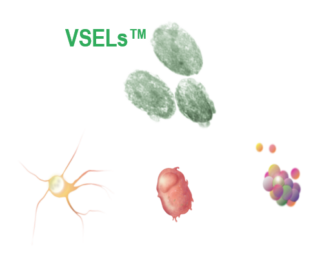
“VSEL” TECHNOLOGY HAS THE POTENTIAL TO REPAIR DAMGAED TISSUE
In-licensed from the University of Louisville the world-wide patent rights and know-how regarding the isolation, purification and therapeutic use of very small embryonic-like (VSEL™) stem cells
- Evaluating the potential of very small embryonic-like stem cells (VSELs)
- Research suggests multi-potency and multi-lineage differentiation into all basic cell types
- Exploring the development for tretinal repair and the treatment of chronic wounds
- $4.5 Mill of grants toward precliniclia VSEL research
Increases manufacturing capacity for Internal Pipeline for the Cell Therapy Industry
- High quality manufacturing capabilities with 15 year track record of success
- Proven efficiencies and reduced capital investment for customers through outsourcing
- Deep regulatory expertise
- 50+ EI+U and US filings
- Trial phases including BLA submission and FDA product approval
- Innovative engineering and automation
- EU product distribution compliant
- Expanding commercial capabilities in the US and Internationally
DEVELOP NOVEL PROPRIETARY CELL THERAPY PRODUCTS
-
Leverage unique capabilities for cost-effective in-house product development
-
Partner select programs at key infliction points
-
Grow pipeline and capabilities through strategic acqusitions
EXPAND REVENUE-GENERATING SERVICE BUSINESS
-
Grow client base organically and through new service areas
-
Expand manufacturing in the US and Internally
-
Expand into cell therapy tools and technology market
Caladrius’s Passion Grows
Today, CBLS leverages its revenue-generating platform of expert capabilities for the manufacture of cell therapies across multiple internal and client development programs, and with each new clinical and regulatory milestone we and our peers in the industry achieve, our passion and excitement about the potential for cell therapy grows. The Caladrius story is the story of experienced management, dedicated employees, industry-leading expertise and a revolutionary spirit hard at work to make the potential of cell therapy a reality.
Download the Caladrius Corporate Profile and Investor Presentation below by clicking on the graphic.
|
|
 |
|
Key Milestones
2014 |
|
2013 |
|
2012 |
|
2011 |
|
2010 |
|